Roche’s bruton’s tyrosine kinas blocker fenebrutinib has met all endpoints in proof-of-concept study with patients suffering from relapsing-remitting multiple sclerosis.
ADVERTISEMENT
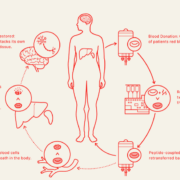
uniQure N.V. is to sell a portion of its royalty rights from the net sales o its hemophilia B gene therapy etranacogene dezaparvovec-drbl to HealthCare Royalty and Sagard Healthcare for a gross purchase price of up to $400m in cash. This financing provides uniQure with immediate, non-equity-dilutive capital to continue to invest in our AAV gene therapy pipeline and platform and to advance potentially breakthrough gene therapies for patients, including AMT-130 in Huntington’s disease, AMT-260 for refractory temporal lobe epilepsy, AMT-162 for SOD-1 ALS and other product candidates, statedMatt Kapusta, CEO of uniQure.
Etranacogene dezaparvovec-drbl is a gene therapy that reduces the rate of abnormal bleeding in eligible people with hemophilia B by enabling the body to continuously produce factor IX, the deficient protein in hemophilia B. It uses AAV5, a non-infectious viral vector, called an adeno-associated virus (AAV). The AAV5 vector carries the Padua gene variant of Factor IX (FIX-Padua) to the target cells in the liver, generating factor IX proteins that are 5x-8x more active than normal. These genetic instructions remain in the target cells, but generally do not become a part of a person’s own DNA. Once delivered, the new genetic instructions allow the cellular machinery to produce stable levels of factor IX.
Under the terms of the agreement, uniQure will receive an upfront cash payment of $375m in exchange for the lowest royalty tier on CSL Behring’s worldwide net sales of etranacogene dezaparvovec-drbl up to 1.85 times the purchase price until June 30, 2032 or, if such cap is not met by June 30, 2032, up to 2.25x the purchase price through December 31, 2038.
Amsterdam-based uniQure NV led the multi-year development of etranacogene dezaparvovec-drbl and completed an exclusive global license and commercialization agreement with CSL Behring in May 2021. Etranacogene dezaparvovec-drbl HEis approved for the treatment of hemophilia B in the US, Europe and the United Kingdom. CSL Behring also is pursuing registration in additional countries.
uniQure expects the transaction will extend its cash runway into the second quarter of 2026, assuming receipt of the contractual milestone payment for the first sale of etranacogene dezaparvovec-drbl in the US.
Belgian T reg specialist Dualyx NV had raised €40m to progress its three preclinical Treg candidates for autoimmune diseases into the clinic.
NHS research partners of AstraZeneca and Sanofi have reported a 83% reduction in RSV infant hospitalisations in a post-approval clinical trial.
Molecular diagnostics specialist Mainz Biomed NV has inked a research collaboration with precision microbiome firm Microba Life Sciences Inc.
French pancreatic beta-cell modulator specialist DiogenX SA has raised €27.5m in a Series A financing led by Boehringer Ingelheim Venture Fund and Roche Venture Fund.
Munich-based Wacker Group has purchased all shares of the Spanish CMO ADL BioPharma from financial investor Kartesia for €100m.
Swedish AI specialist Neogap Therapeutics AB will secect antigens for Cellerys AG, which wants to establish immune tolerance in a Phase II MS trial.
The State of the Netherlands, and the Dutch Ministry of Health, Welfare and Sport will sell all shares of vaccine maker Intravacc B.V.
Tay Therapeutics Ltd announces licencing of its oral BET blocker TayB2 to VYNE Therapeutics Inc as a treatment for immuno-inflammatory and fibrotic disorders.