The decision of the vaccine arm of French drug giant Sanofi was based on an independent interim analysis of a pivotal Phase III study enroling up to 16,500 people at risk of Clostridium difficile infection (CDI) for vaccination with the jab that has FDA Fast Track designation.
ADVERTISEMENT
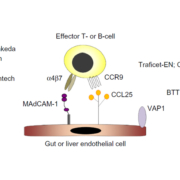
Shire has received FDA Orphan Drug Status for its anti-MAdCAM Antibody SHP647 to treat pediatric ulverative colitis.
845 medical affairs and biopharma industry professionals at Veeva Commercial & Medical Summit Europe in Madrid discussed how digitalisation can speed up the development of new medical solutions while reducing cost.
Belgian liquid biopsy specialist Biocartis Group NV has pocketed €80m through an equity placement by offering 6.4 million new shares.
French green chemistry specialist Global Bioenergies announced the delivery of a first batch of sustainable cosmetic ingredients to cosmetics heavyweight L’Oréal. It was produced as part of the ISOPROD project, supported by the French State’s Investissements for the Future Program.
Three innovative biotech companies have baged the prestigious EuropaBio SME Awards for their innovative business models.
The same bacteria present in primary tumours of patients with colorectal cancer are also present in liver metastases, according to a new study. What’s more, presence of the bacteria was found to correlate with tumour growth.
The EU is investing another €6.3m into the blood diagnosis of primary breast cancer and rectal cancer. In the 4-year LIMA project, Royal Philips will lead a SME consortium that combines liquid biospy of circulating tumour cells (CTCs) with Magnetic Resonance Imaging (MRI).
Antibody-drug-conjugate (ADC) specialist Heidelberg Pharma AG has raised €34.4m through the issuance of 7,484,190 new shares at €2.60 and 14,968,380 convertible bonds with a nominal value of €1 each. Baader Bank AG acted as global coordinator and sole bookrunner.
Amsterdam has beaten Milan and Copenhagen in the race to become the European Medicines Agency’s new home. Pharma and biotech stakeholders applauded the vote as Amsterdam achieved the hightest rate of acceptance of the EMA staff for relocatation, according to a survey.