Alkermes plc (Dublin) received a US$50m milestone payment from Biogen after review of preliminary Phase III gastrointestinal tolerability data of Alkermes’ relapsing-remitting multiple sclerosis candidate diroximel fumarate (BIIB098).
ADVERTISEMENT
Austrian arenaviral vector technology specialist Hookipa Biotech AG (Vienna, Austria) has licenced its TheraT and Vaxwave technologies exclusively to Gilead Sciences Inc. to develop and globally commercialise vaccines against the human immunodeficiency virus (HIV) and the hepatitis B virus (HBV).
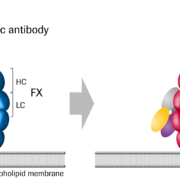
The US FDA has accepted a supplemental BLA to Roche’s existing market approval of the new hemophilia A treatment Hemlibra (emicizumab), which could significantly extend the patient group covered by the label.
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended nine medicines for approval, including two orphan medicines, at its May 2018 meeting.
French researchers have found a key driver of chronic epilepsy.
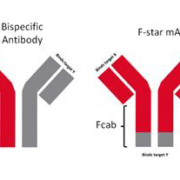
Brain disease specialist Denali Therapeutics will buy all outstanding shares in British F-Star Gamma Ltd from F-Star instead licencing bispecific antibody candidates that can cross the blood-brain barrier (BBB) co-developed under an agreement dating back to 2016.
Roche announced that atezolimumab plus chemotherapy met its co-primary endpoints of overall survival (OS) and progression-free survival (PFS) as first-line treatment of stage IV nonsquamous non small cell lung cancer (NSCLC).
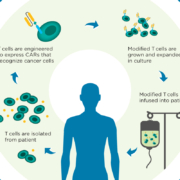
Interleukin 1 (IL-1) blockers may prevent serious adverse events triggered by CAR-T cell therapies, according to two independent studies.
Following reports of an increase in mortality in a study that combined Genmab’s anti-CD38 daratumumab with a PD-L1 blocker, Genmab’s partner Janssen has stopped a study combining daratumumab with its PD1 blocker JNJ-63723283 in multiple myeloma.
Targovax ASA’s cancer immunotherapy TG01 significantly prolonged 2-year overall survival of patients with resected pancreatic cancer versus standard therapy. Targovax’ shares were up 16% on Thursday evening.