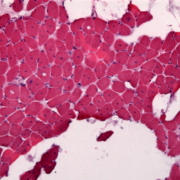
UK biotech Oxford BioDynamics has presented data showing that the company’s blood test can predict patient response to treatment with immune checkpoint inhibitors anti-PD-1 and anti-PD-L1.
ADVERTISEMENT
Swiss biopharma company Urovant Sciences has launched its IPO on Nasdaq, seeking US$150m. The proceeds will go to Phase III trials for its overactive bladder treatment candidate vibegron.
Danish Genmab and German Immatics Biotechnologies are collaborating to discover and develop next-generation bispecific immunotherapies to develop therapeutics in multiple cancer indications.
Using only openly available genomic data, researchers in Barcelona have developed a new method to systematically identify genes contributing to heritable cancer risk.
Norwegian diabetes expert Lifecare AS has gone public on the Oslo stock exchange. The sensor maker plans to fund the next phase of development for its implantable glucose sensor.
Bioscience investment firm Abingworth has closed its latest fund at US$315m (€268m). The money will go to life sciences companies in Europe and the US.
After €7m went to Nextbiotix last week, another financing round is bringing money to a young French biotech. Strasbourg-based Dynacure attracted €47m from investors to bring its lead candidate for centronuclear myopathy into clinical development.
Microbiome start-up Nextbiotix raised € 7m in a Series A round. The Paris-based biotech uses commensal bacteria as drugs to treat major inflammatory bowel diseases.
Dialysis product and service provider Fresenius Medical Care has placed €500m notes. Yesterday, the medtech giant had to come to terms with a sharp drop in share price.
The 33rd edition of Chemspec Europe, International Exhibition for Fine and Speciality Chemicals, ended after two successful show days with record visitor numbers. From 20 to 21 June 2018, a total of 4,202 trade visitors came to the exhibition grounds of the Koelnmesse in Cologne, Germany, where international experts of the fine and speciality chemicals industry met for Europe’s leading exhibition. These figures represent an increase of visitor numbers by nearly 9% compared to last year’s show in Munich.