Seventure Partners has announced the first close of its second microbiome fund designed to put €200m in 20 companies developing microbiome-based health solutions.
ADVERTISEMENT
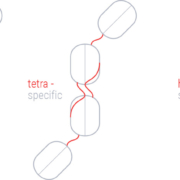
Swiss immunology and immuno-oncology specialist Numab Innovation AG has licenced its tri-specific antibody ND016 to Boston-based Intarcia Therapeutics Inc.
Biogen has strengthened its portfolio with Nightstar Therapeutics, a British gene therapy specialist for rare inherited eye disorders.
The EMA‘s human medicines committee (CHMP) has recommended eight medicines for approval in its last meeting in London, two thereof orphan medicines. Three got conditional marketing authorisation.
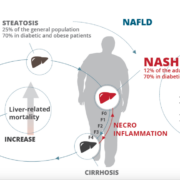
French Genfit SA has registered at SEC for an Nasdaq IPO of ADS in conjunction with private placements in Europe and the US.
Researchers from the UK and the US have prevented tendon injury and degeneration by simply blocking a key signalling pathway in inflammation.
University Zurich spin-off Anaveon AG announced it will proceeds from its financing round to push development of its preclinical IL-2-receptor agonist.
The global antibacterial innovation network CARB-X has expanded its reach by adding six new life-science organisations to its fight against antimicrobial resistance (AMR).
Swiss pharma giant Roche has complemented its drug pipeline in the lucrative hemophilia A market by taking over hemophilia A gene therapy developer Spark Therapeutics for US$4.3bn.
Sodium chloride can promote immune responses that have been linked to atopic dermatitis. Researchers have now unraveled the mechanistic details between salt intake and activation of T cells.