Boehringer Ingelheim has licenced a preclinical fusion protein from Korean Yuhan Corp. that activates both GLP-1R and FGF21R to treat Nonalcoholic Steatohepatitis (NASH). Boehringer Ingelheim will pay $40m upfront and in near-term payments to acquire the commercialisation rights to the compound. Under the agreement, Yuhan Corp. is eligible to receive up to USD 830 million in potential milestone payments plus tiered royalties on future net sales.
ADVERTISEMENT
A French-Swiss research team is developing a smart bra for detecting breast cancer more accessible than through mammography.
GARDP has called up the global community to develop and deliver five new treatments that break antimicrobial resistance (AMR) by 2025.
Sanifit has bagged €72.2m in Spain’s largest private biotech fundraise.
Genfit SA has licenced the Greater China commercialisation rights for its NASH drug elafibranor to Terns Pharmaceuticals Inc.
Life sciences venture capitalist Vesalius Biocapital III has announced the final close of the VBC III fund at €120m.
Erytech Pharma has inked a deal with SQZ Biotechnologies Inc. to develop new immune modulating therapies.
Sanofis and Regenerons IL-33 blocker REGN3500 met primary Phase II endpoint in asthma patients.
Karo Pharma AB announced it will acquire Trimb Holding AB for MSEK3,400 (€319.6m) and intends to carry out a Rights Issue of about MSEK 1,500 (€141m).
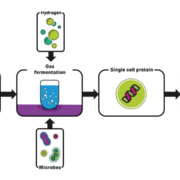
Deep Branch Biotechnology Ltd. from Nottingham has started a feasibility study with the British energy supplier Drax Group. The technology to be analysed converts carbon dioxide emitted by power stations into protein that could be used for feeding fish and livestock.