In silico clinical trials pioneer Novadiscovery SA has raised €5m in a series A financing led by Swiss Debiopharm Innovation Fund.
ADVERTISEMENT
Following a €1.5m seed financing in 2017, cancer specialist Alderaan Biotechnology SAS has raised €18.5m in a Series A round led by Advent France Biotechnology and Medicxi.
NorthSea Therapeutics BV has raised US$40m to provide proof-of-concept of its NASH drug icosabutate.
4SC AG and Merck KgaA have signed a supply agreement to start clinical tests of Merck’s checkpoint blocker avelumab plus 4SC’s HDAC I blocker domatinostat.
Mutabilis and IBT Vaccines have successfully bagged US$12m from the Novo Holdings REPAIR Impact Fund.
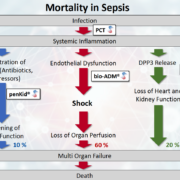
Critical care experts have presented data supporting the thesis that three specific pathways are the cause for about 90% of sepsis mortality.
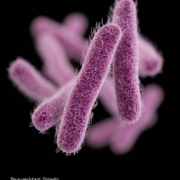
Antabio SAS has got a US-$4.4m tranche from CARB-X to advance preclinical development of its P. aeruginosa treatment in Cystic Fibrosis patients
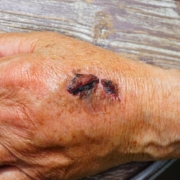
Swedish and Danish researchers have created a hydrogel that kills various types of bacteria and reduces inflammation in mice and pigs with infected wounds.
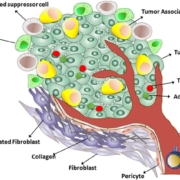
The technology of Oxford-based Oxford Biodynamics (OBD) promises insights into why immunotherapies only work in some patients but not in all. A major US pharma company now wants to validate OBD’s epigenetic biomarker discovery and development platform causing a share price gain of 15%.
Paris-based European venture capital company Truffle Capital closed its €250m BioMedTech fund – celebrating its company building approach.