French Abivax SA has received a €36m non-dilutive funding from Bpifrance for its ABX464 Covid-19 programme.
ADVERTISEMENT
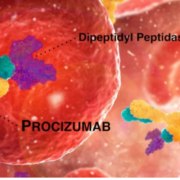
4TEEN4 Pharmaceuticals GmbH published data suggesting that high plasma levels of dipeptidyl peptidase 3 were indicative for upcoming multiple organ failure in burn patients.
The funding pot of the Innovation Medicines Initiative has been boosted to €117m. IMI has also selected eight projects to fund.
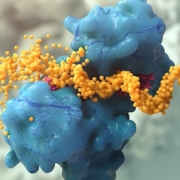
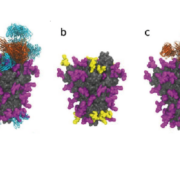
British researchers have deciphered the glycosylation pattern of the viral spike protein of SARS-CoV-2 laying the foundation for vaccine development.
Newron Pharmaceuticals S.p.A.s Sarizotan failed to meet efficacy endpoints in the Phase III STARS study.
Roche Diagnostics has received FDA Emergency use approval for its highly specific Elecsys antibody test.
Belgian Bone Therapeutics SA has secured €11m to foster development of its late stage pipeline candidates.
Cevec Pharmaceuticals GmbH has launched a new, scalable production platform for AAV gene therapy vectors.
German vaccine developer ARTES Biotechnology GmbH will develop a COVID-19 subunit vaccine based on virus-like particles (VLP), which include two proteins of SARS-CoV-2.
The politically overhyped use of old malaria drugs in combination with COVID-19-specific candidates has been demonstrated to be dangerous, reports Nature Medicine.