After leaving the field of antimicrobials 20 years ago because other areas offered better returns, 23 pharma companies and the Novo Foundation have launched a US$1bn fund to fight antimicrobial resistance (AMR).
ADVERTISEMENT
Leiden-based coagulant developer VarmX NV has closed a €32m Series B financing to push clinical development of VMX-C001, a venom-based modified recombinant Factor X.
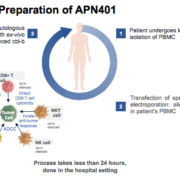
Viennese APEIRON Biologics and MaxCyte Inc have entered into licensing agreement covering the use of Maxcyte’s electroporation platform for APN401 development.
After closing the business merging with blank check company Arya Sciences Acquisition Corp, Immatics Inc has been listed on Nasdaq.
A new insulin formulation allows patients with diabetes to more quickly manage their blood sugar levels during mealtimes.
Data from the UK’s RECOVERY trial demonstrate there was no clinical benefit from use of lopinavir-ritonavir in hospitalised COVID-19 patients.
Under the Coronavirus Global Response Framework, the European Commission is allocating further €4.9bn to assure access to COVID-19 countermeasures for poor countries.
BioGeneration Ventures has closed its fourth VC fund for European start-ups (BGV IV) at €105m.
OneProjects Ltd, an Irish-German cardiac imaging specialist, has closed an €11m Series A financing led by Amsterdam-headquartered life sciences investor LSP.
Illumina has opened its first accelerator outside the US in Cambridge to fund genomics start-ups. Meanwhile, Epidarex Capital has raised £102m for UK biotechs.