Setback for CureVac: Boehringer Ingelheim halts a 7-year-old €500m lung cancer collaboration with the Tübingen-based biopharmaceutical company.
ADVERTISEMENT
Vaccine maker Valneva SE announced the initiation of a further Phase III trial for its COVID-19 vaccine candidate VLA2001.
Visus Therapeutics has baged a €20m stock financing to advance development of Brimochol for presbyopia
The protein eIF4A3 may hold the key for future cancer treatments, Karolinska researchers find.
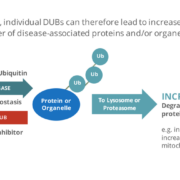
British drug developer Mission Therapeutics plc has bagged a $20m milestone payment from Abbvie within the companies’ neurodegenerative disease collaboration.
The big pharma company is shelling out US$1.5bn in the acquisition of US screening specialist Vividion Therapeutics.
Belgian semiconductor specialist Spectricity BV has baged €14m to speed up the development of mobile hyperspectral sensors for medical applications.
Sanofi further launches into the messenger RNA space with US$3.2bn buy-out of mRNA pioneer Translate Bio.
Life sciences tool provider Abcam plc has entered into a definitive agreement to acquire BioVision, Inc. for $340m.
Cancer and autoimmunity specialist Byondis BV and carbohydrate-targeting antibody company Glycotope have entered into a platform access agreement.