Your mRNA Partner in Europe
Messenger RNA (mRNA) has gained significant attention since being used in the Pfizer-BioNTech and Moderna COVID-19 vaccines. mRNA holds potential for preventing and treating many difficult-to-treat or genetic diseases, including cancers. However, its production is complex and raises challenges for researchers developing new therapies: Discover Tebubio.
Demand for mRNA has skyrocketed and researchers developing mRNA-based vaccines and therapies face the optimisation of stability, immunogenicity, translation efficiency, and delivery. EU-based Tebubio Contract Research Services provides mini-scale mRNA production to support proof of concept work. It produces μg to mg amounts of custom mRNA in Europe, to be used for screening purposes in preclinical research. This unique, personalised, mini-scale mRNA optimisation and production service guarantees support for researchers from start to finish, with successful transfer to scale-up with the end product in mind, from the outset.
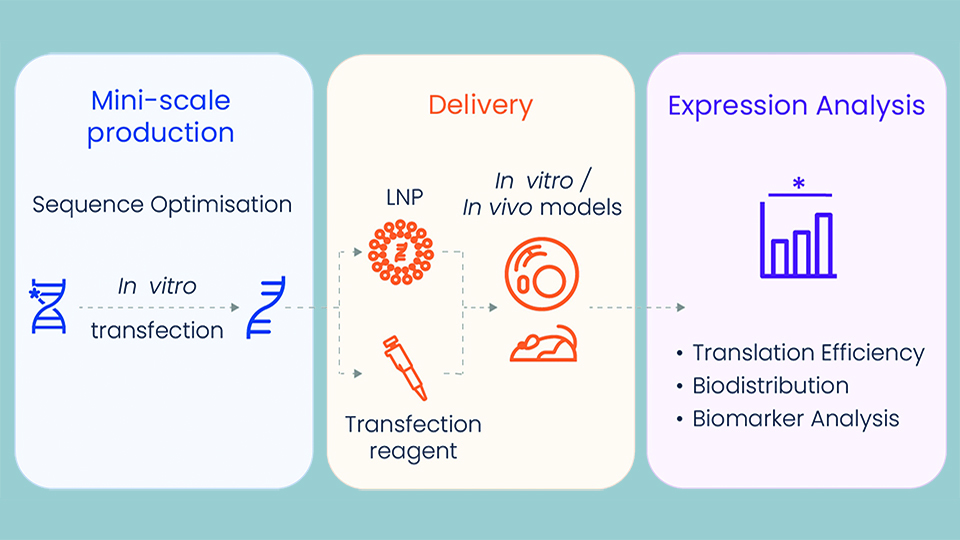
Mini-scale production
Researchers no longer struggle to get a functional mRNA sequence. Having completed more than 300 projects in the last three years, Tebubio experts are skilled in optimising the template pDNA sequence to increase its stability, transcription efficiency, final mRNA and protein expression, and decrease immunogenicity. Once the sequence is optimised, research grade mRNA can be produced as of 100 μg at Tebubio’s dedicated, state-of-the-art mRNA laboratory in France.
Delivery
Intracellular delivery of mRNA represents a challenge, due, in part, to its large molecular weight and high negative charge density. Customised delivery tools are required to suit the specific requirements of the final model (target, tissue, cells, disease). Tebubio employs either chemical delivery with a transfection reagent, or customised lipid nanoparticle formulations. With the knowledge and expertise to develop the best formulation, Tebubio streamlines production and provides mini-scale quantities of formulations, that are scale-up-ready.
Expression & analysis
Tebubio validates the expression of the mRNA in the chosen in vitro model, whether in cells, 2D and 3D culture, or on in vivo models. Further analysis can include protein expression, quantification and localisation, and the response of the in vitro models, such as: toxicity, inflammatory cytokine release, cytokine storm, phosphorylation, and secretome profiling. Once the encapsulated mRNA has been optimised, Tebubio provides seamless handover to trusted partners to develop the in vivo models, with close support provided by a PhD-level Tebubio project manager throughout. All of the pre-clinical variants are validated in vitro, which reduces the number of candidates needed for in vivo testing, prior to clinical trials. Furthermore, Tebubio’s in-house biostatistics platform delivers complete biomarker analysis, with reports including robust proof of concept data that can be used in publications and grant/patent applications.
Upscale + GMP
At Tebubio, mRNA is designed with the final product in mind and every tool used is GMP-compatible. Support is provided for a smooth transition to larger manufacturing solutions.
To accelerate mRNA research projects, contact one of Tebubio’s local offices: www.tebubio.com/contact
Author: Flavien Carpentier, Contract Research Services Director, Tebubio SAS, Le Perray-en-Yvelines, France
This article war originally published in European Biotechnology Magazine Autumn 2024.


 adobe stock photos - AkuAku
adobe stock photos - AkuAku