Vaccine production in Africa: an urgent priority
Africa produces less than 1% of the human vaccines it uses. To effectively respond in time of outbreaks and pandemics, Africa must expand its vaccine development and production capabilities to cater for its growing population. Successfully establishing such an industry at scale is a multifaceted endeavour to create an enabling environment. This article highlights key factors and considerations for establishing local vaccine manufacturing.
The African vaccine market is forecasted to grow at approximately €2bn to €4bn by 2030. With limited vaccine manufacturing capacity and infrastructure, Africa largely depends on external supply and is particularly vulnerable during pandemics. Establishing a supportive environment for vaccine manufacturing is possible and requires long-term strategic thinking guided by a clear vision.
Development and manufacture of vaccines in Africa will create long-term benefits in terms of health impact, socio-economic and industrial development, development of biotechnology skills, and advancement of the life sciences in general.
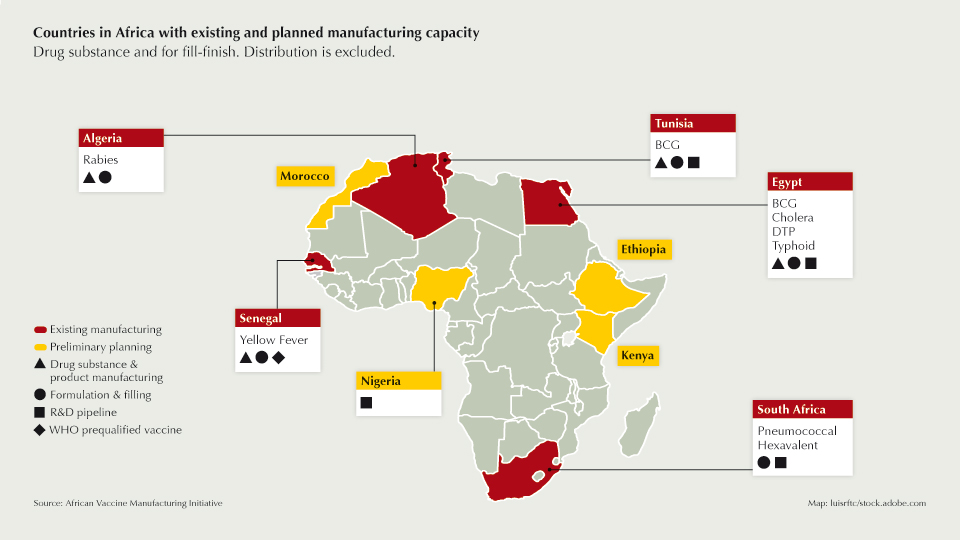
Realising this vision will require tangible and long-term commitment from governments across the continent, individually and collectively. Countries have existing capacity, which could be modernised and expanded while others have preliminary plans (see map). Key factors such as economy of scale, regulatory frameworks, long-term business sustainability, manufacturing know-how and access to technology must be addressed. Building capacity depends on focused political and technical support, including a coherent and well-funded regional policymaking and planning approach.
Innovative approaches to financing from the public, private, investor and international agencies or NGOs will be crucial. Public-private partnerships, enabling markets and diversification in both investments risks and product portfolio are also essential.
Regulatory frameworks
Strengthening local National Regulatory Agencies (NRA) to enable local manufacturing should be a priority to ensure efficacy and the safety of vaccines. Several NRAs are operational, and efforts are underway to strengthen quality management systems, clinical trials assessment, pharmacovigilance and technical skills of the inspectors.
Notably, the World Health Organization (WHO) initiated the African Vaccine Regulatory Forum (AVAREF) in 2006. This forum supports NRAs in making informed decisions about authorisation of clinical trials, evaluation of product registration dossiers and other challenging issues related to vaccine quality. Close collaboration with international regulatory agencies will help ensure success in this critical area.
A major change is expected with the establishment of the African Medicine Agency (AMA) of the African Union, which will enable countries to regulate medicine and increase access to quality and potent products. To date, the treaty for the AMA has been ratified by eleven member states.
Development & Manufacturing
Africa’s current infrastructure needs to be expanded and facilities modernised to diversify the continent’s manufacturing capabilities and increase capacity for vaccine production. Biovac in South Africa is using reverse integration where establishment of capability starts at the end of the vaccine manufacturing value chain and works upstream over time. In this way, Biovac has steadily progressed from cold chain warehousing and distribution to labelling and packaging of vials to sterile filling and formulation of vaccines. Reverse integration is a first step towards fully integrated manufacturing capability.With an expected population of 2,4 billion by 2050, Africa must consider multiple facilities to produce critical vaccines and ramp up capacity in case of a pandemic. A new facility would require between €20m to €200m investment depending on the target capacity and the selected technology for manufacturing (stainless steel, single-use, hybrid). According to a study from the African Vaccine Manufacturing Initiative (AVMI) and UNIDO, a minimum of 10 million doses is needed to ensure business sustainability. Large facilities producing more than 300 million doses can justify the use of stainless-steel equipment due to the high volumes needed upstream. Manufacturing with single-use equipment can be competitive for output below 300 million doses. Flexible manufacturing offers the potential for multiple vaccines to be processed in one facility. The result can be a more rapid response to demand. In the graphic above, the cost of manufacturing a single vaccine using single use/hybrid or stainless-steel equipment is compared. A single use/hybrid facility would allow a 54% increase of capacity and 36% decrease in cost of manufacturing per dose, despite the increase consumables cost.
As a first step, Africa will need to plan for fill/finish capacity across the continent. As observed during the COVID-19 crisis this is a bottleneck. Several contract manufacturing organisations have been able to respond to this need, even those that have never manufactured vaccines, using installed capacities, which could be repurposed at short notice. It is also important to note that the availability of innovative platforms such as viral vectors or nucleic acids that allow for accelerated and more cost-effective vaccine development and manufacture will play a significant role.
Finally, Africa must establish a vaccine R&D strategy to ensure products that suit Africa-specific disease burdens are developed. The emergence of the COVID-19 501Y.V2 variant in South Africa is a case in point. Vaccines are being developed on the continent; we can cite a novel Group B streptococcus vaccine in South Africa, Rabies vaccine in Tunisia and COVID-19 vaccine activities in Nigeria and Tunisia. These efforts should be built upon and expanded through investments of time, effort and money supported by long-term political will. Development of a highly skilled workforce from R&D to manufacturing also needs to be a priority as well as engagement with global vaccine R&D groups.
The time for change
The COVID-19 pandemic is a wakeup call for Africa and, in the face of vaccine "nationalism", the time has come to build vaccine development and manufacturing capacity to increase self-reliance without further delay. Paradigms need to change. Urgent action is necessary. Immediate and focused strategy and actions to accelerate the rollout of large-scale vaccine development and manufacturing capabilities will impact Africa for generations to come. Partnerships among key stakeholders with a broad range of expertise and the potential to unlock resources should be expedited. Champions should be identified to mobilise national and regional efforts.
Indeed, the challenges are significant, however, the costs, financial and otherwise, of not advancing vaccine development and manufacturing capacity in Africa is far greater.



 Unsplash+
Unsplash+