Preventing congestion in acute heart failure patients
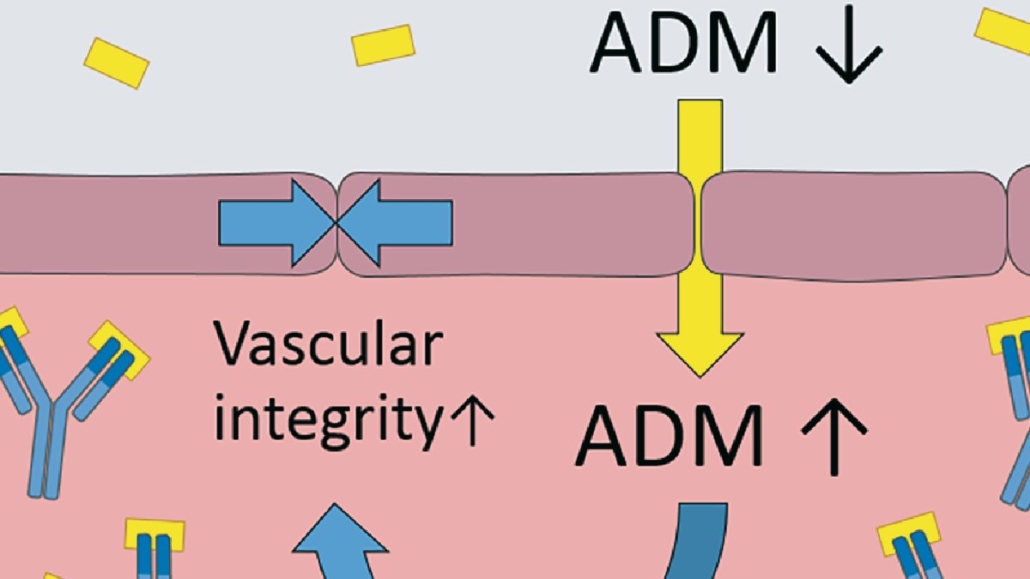
According to Adrenomed, preclinical and Phase I data suggest a unique mode of action of adrecizumab. The antibody leads to redistribution of the peptide hormone adrenomedullin (ADM) from tissue into blood vessels without affecting its activity. There, it closes the gaps in the endothelial layer of blood vessels, which contribute to congestion. Up to now, the leakage of fluid from the blood into lungs has been treated with diuretics. However, most of the annual treatment cost of US-$39bn for congestive heart failure is due to patients refractive to diurectics who develop pulmonary edema and currently can’t be identified before hospital admission. Adrenomed is set to solve the problem.
Stratification first
In sepsis patients, the hoover effect leads to lowering of their extremely high adrenomedullin tissue levels, which prevents the drop in blood pressure preceding septic shock. Phase II trials in sepsis and congestive heart failure are expected to be kicked off in H1/2017. Big pharma companies have already contacted the company for more information.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink