Blow-Fill-Seal technology and vaccine delivery
Unit-dose?The SARS-CoV2 pandemic caused 6.3 million deaths and contaminated 545 million people across the world. In spite of the rapid development and approval of vaccines, the shortages of consumables like glass vials limited the distribution of prophylactic vaccines. Blow-Fill-Seal (BFS) is an alternative solution with advantages that could play an important role in vaccine distribution worldwide.
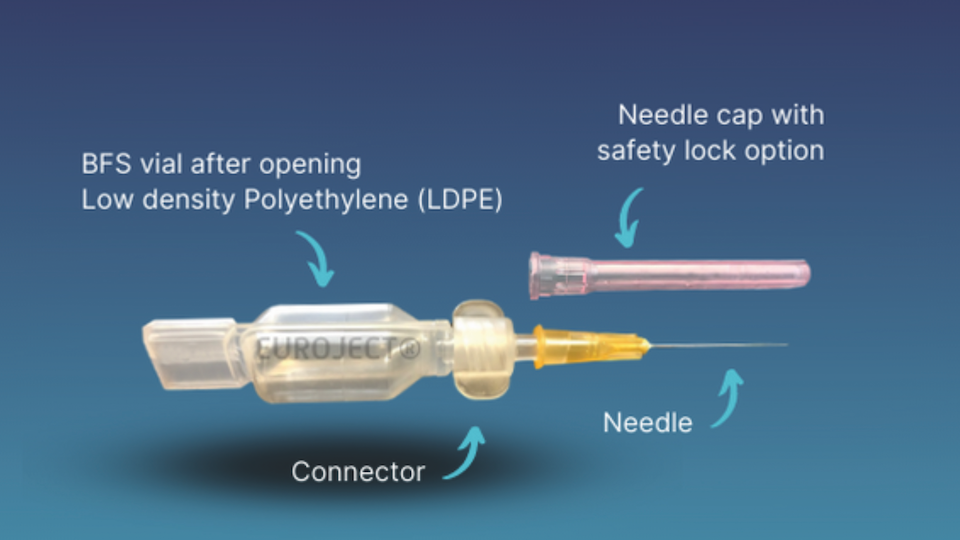
In this context of sanitary crisis, Unither Pharmaceuticals, a BFS leader with almost 30 years of experience, launched Euroject® in Sept. 2021 combining a BFS unit-dose vial with a needle hub for vaccine injection. This new device goes with a dedicated BSL2 workshop, based in Amiens (FR), which will be able by 2024 to fill 1 billion doses annually. Meanwhile, all internal and customer development tests are performed on an industrial full-size BottlePack (BP) 460 machine in Coutances (FR).
BFS history
BFS technology is originally derived from a 1963 patent application. At that time, the concept allowed the packaging of food, cosmetics, and medical device products, all in non-sterile condition.
The first BP machine was launched in 1964. In the early 1970s, the pharmaceutical industry used the equipment to pack large volumes of pharmaceutical solutions. (Oschmann & Schubert, 1999). At the end of 1980s, the BFS technology was well-established in the packaging industry, particularly for pharmaceutical and healthcare products. Along the 1980s and 1990s, BFS was developed and is now adopted for the unit-dose format of sterile solutions. Throughout early 2000s, BFS has been used for parenteral products instead of glass bottles because it is light and unbreakable. In the final EU GMP Annex 1 on the manufacture of medicinal products, published in August of this year, BFS is one of the essential manufacturing processes for sterile products. Since multiple vials are formed by a mold and filled in a row, and because the machine can have multiple molds at once, the cadency can reach 33,000 vials per hour. Extrapolated over the year, this can lead to the production of 200 to 300 million vials per machine!
BFS is adapted to vaccines
In the BFS process, the plastic polymer granules are melted at high temperatures, around 160 °C, then extruded and blown into a vial shape. During the shape formation, a needle fills the dose with the drug product before sealing and demolding the vial. The entire process is fully automated and performed in aseptic environment.
At first glance, the process might seem incompatible with temperature-sensitive molecules like biologics and vaccines. Indeed, the temperature is high enough to damage a biologic’s function when exposed for too long. In fact, the full BFS process cycle is fast, only a few seconds, limiting drastically the exposure to deleterious temperature.
In the past, BFS vials have been used as primary container of vaccines, even at commercial stage. In early 2010s, MedImmune conducted a Phase III clinical study to evaluate the immunogenicity of an intra-nasal influenza vaccine (MEDI8662) supplied in BFS compared to FluMist®/B, the reference supplied in a Becton Dickinson Accuspray device. The results showed that the BFS live-attenuated vaccine is immunogenically non-inferior to the reference and the adverse effect were comparable.
More recently, the vaccine against human rotavirus, Rotarix® from GSK, still a live-attenuated virus, used a BFS vial for the packaging of the vaccine bulk to improve access to this oral vaccine in low-and middle-income countries, like in Myanmar where multidose BFS containers are used. No matter the container, including glass vials, the stability of this vaccine over 12 months is comparable (Manjari, et al., 2016).
These examples prove that the BFS process did not affect the efficacy and the storage of the vaccine even if considered temperature sensitive.
Regardless, the performance of the primary container and its compatibility with the product must be demonstrated over the vaccine’s shelf life, and this stability must be evaluated through a production-scale filling process. In addition, the cost to produce the vaccine in each considered container must be taken into consideration. This exercise has been done by PATH for oral (Rotarix) and parenteral vaccine (IPV) (Jeff, et al., 2018). It shows that BFS is the best option for oral vaccine and just below the 10-dose glass vial for parenteral route. However, this study does not take into consideration the potential issues of cross-contamination that can be dramatic for the population, considering that in many cases it is a prophylactic vaccination for healthy people.
Considering the advantages of BFS compared to the other solutions, the Euroject® device has been designed to respond to the Fill&Finish of biologics and vaccines for an accurate and a cost-effective delivery.
We optimized the set-up of the bottlepack (BP) machine and its peripheric to maintain the biologic in an appropriate environment. This setup has been tested for the filling of a surrogate vaccine (Arturo, et al., 2021). To do so, we prepared a bulk of 3L containing 60 mg of SARS-CoV-2 RBD antigen. The industrial BP machine was run to dispense 0.5 ml of product per dose. The full run took less than 7 min. and produced 6,000 vials! To evaluate the filling process impact on the molecule, samples were taken along the run and were analysed by different techniques for biochemical and functional properties. The samples were compared to a reference, which was the same product before the run.
The experimental size of the RBD protein, evaluated by SDS-PAGE or SEC-HPLC, was shown to be preserved with a mass of 30 kDa and a retention time of 27.5min. The protein mass at the end of the run was 27014.6 Da and was comparable to the reference when analysed by LC-MS after reduction and PNGase-F treatment. Similarly, no degradation or aggregation were detected by SDS-PAGE or HP-SEC analysis. Biochemical characterisation was completed by nano-DSF analysis giving a Tma value between 51.8 to 52.4 °C for the samples compared to 51.9 °C for the reference, showing that the process did not impact the folding of the molecule. This was confirmed by ELISA, where the binding of RBD to E2 was conserved.
Altogether, the literature completed to our data with the SARS-CoV-2-RBD antigen shows that the BFS process, even if it involves high temperature, is clearly compatible with biologics and vaccines. One question could remain on the new class of vaccines based on mRNA. In absence of data, we believe that mRNA is compatible with BFS and the temperature is not an issue. Indeed, previous studies have shown that the mRNA vaccine against rabies can resist a temperature of 70°C for three months (Lothar, et al., 2017). This is largely higher than what can face a molecule in BFS process optimized for biologics at Unither.
BFS is largely adopted in the pharmaceutical industry as primary container for storage and delivery of liquids. At Unither, we are deeply convinced that BFS unit-dose and Euroject® technology are part of the solution to store and deliver biologics and vaccines. Our R&D offer and feasibility testing combined with our new capacities will contribute to the international effort to deliver drug products to the global population, by limiting the impact of primary container shortage, to better protect the world population in case of a pandemic.


 Unsplash+
Unsplash+
 Unsplash+
Unsplash+