Ose and Boehringer ink €1.1bn deal in immunoncology
French Ose Immunotherapeutics SA has licenced global commercialisation rights of its preclinical programme OSE-172 to Boehringer Ingelheim which hopes to complement its immunoncology portfolio with a tumour microenvironment modifier that reactivates effector T cell responses.
Under the agreement Boehringer Ingelheim will pay €15m upfront and additional €15m to Ose Immunotherapeutics for starting Phase I testing of OSE-172 by 2019. Furthermore, the contract holds out the prospect to grant €1.1bn in development, commercialisation and sales milestones.
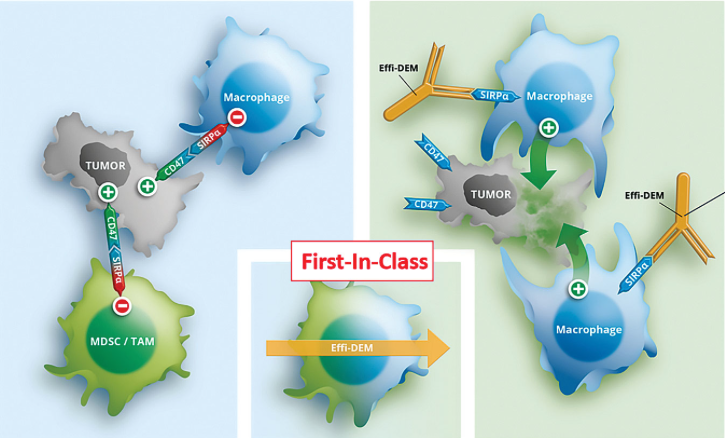
OSE-172 is a monoclonal antibody that disturbs the interaction of CD47 on cancer cells and signal regulatory protein alpha (SIRPalpha, SHPS-1) on myeloid-derived suppressor cells (MDSC) such as non-inflammatory, tumour-promoting M2 tumour-associated macrophages (M2 TAMs) and other phagocytes of the innate immune system turning the immunosuppressive tumour environment into a non suppressive biochemical niche. Furthermore, CD47 provides a downregulatory "don’t eat me"-signal that inhibits host cell phagocytosis. An initial patent application was filed and withdrawn in 2014 by Nicolas Poiriet and colleagues but refiled in 2015 in Europe and is pending. OSE-172 triggers differentiation of a previously not decribes cytotoxic NK cell phenotype.
MDSC modulators, such as Roche’s Phase II CSF1R blocker Emactuzumab (RG7155), which most recently failed to cross the lifecycle investment point (LIP) to Phase III testing, are classical add-on immunotherapies as their effect up to today has not been proven to pronounced enough to show effecacy as a monotherapy.


 Unsplash+
Unsplash+
