New target for lung fibrosis discovered
Canadian and Finnish researchers have demonstrated that long immune cell contact to lung tissue turns tissue repair into fibrosis.
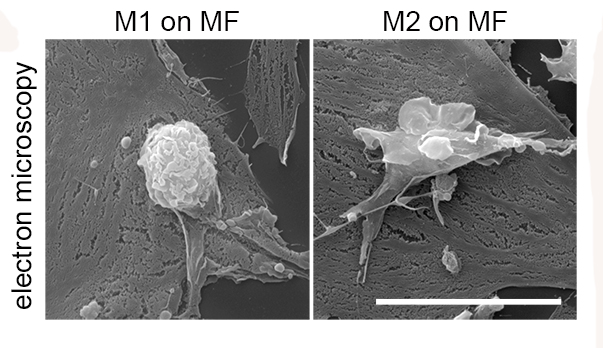
Seeking for the reason why myofibroblasts, which normally secrete collagen in response to inflammatory signals to repair tissue injury in the lungs, turn on fibrotic processes when permanently activated, lead author Monika Lodyga and collegues from University of Toronto and Oulu University Hospital analysed fibrotic lung tissue from mice and men. They discovered that macrophages were persistently attached to myofibroblasts and that contact points between myofibroblasts and macrophages were rich in the protein cadherin-11.
Further analysis showed cadherin-11 acted as a sticking point to keep macrophages and MFs in physical contact, allowing the macrophages to deliver a growth factor named TGF-? that persistently activated the myofibroblasts thereby triggering excessive collagen production that led to accumulation of scar tissue that hinders organ function. Importantly, cell culture experiments showed that direct contact was required for macrophages to switch their phenotype from M1 to M2 macrophages that activate MFs and create a profibrotic niche. The researchers believe that targeting cadherin-11 interactions could help separate myofibroblasts from macrophages and tamp down pro-fibrotic signaling in lung fibrosis.


 ©FabienMalot
©FabienMalot Lonza Group
Lonza Group Vetter Pharma
Vetter Pharma