IL-10R-deficient macrophages trigger IBD
Researchers from Israel and the UK have identified how the cytokine IL-23 triggers inflammatory bowel syndrome (IBD), which affects 1.6 million patients annually in the US alone.
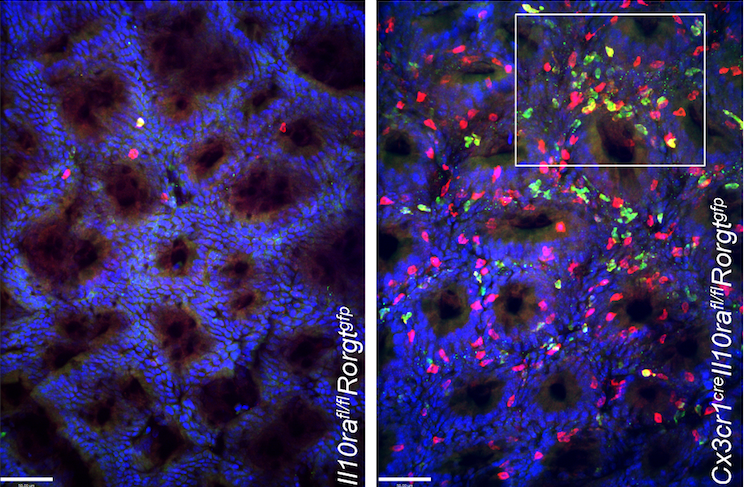
The research team headed by Venizelos Papayannopoulos (Francis Crick Institute, London) and Steffen Jung (Weizmann Institute of Science, Rehovot) discovered that macrophage-made IL-23 triggered the inflammatory phenotype in mice models of the inflammatory bowel diseases (IBDs) ulcerative colitis, and Crohn’s disease. In mice lacking the immune-response dampening IL-10 receptor (IL-10R) protein, mice spontanousely developed symptoms of colitis similar to that observed in children with IL-10R mutations. IL-10 signals are critical for gut homeostasis.
Exploring the effects of the IL-10R mutation in the IBD mouse model, they found that a critical trigger for the colitis-like symptoms was IL-23. IL-23 triggered accumulation and IL-22 production by TH17 cells that, in turn, promoted production of chemokines by colonic epithelial cells and destructive neutrophil recruitment. Interestingly, mice with macrophages deficient in both IL-10R and IL-23 were protected from colitis, supporting the potential for IL-23 as a possible prime target for colitis therapy. The findings are particularly of interest to Janssen Cilag, which markets the psoriasis antibody ustekinumab, which blocks the p-40 subunit of both IL-12 and IL-23 and is also approved as treatment for Crohn’s disease.



 Microbiotica
Microbiotica Araris Biotech AG
Araris Biotech AG