Human cell chip predicts chemotherapy effects
Roche and US company Hesperos Inc have used human cancer and organ cells, placed in a 5-chamber microfluidic chip, to predict chemotherapy safety and efficacy preclinically.
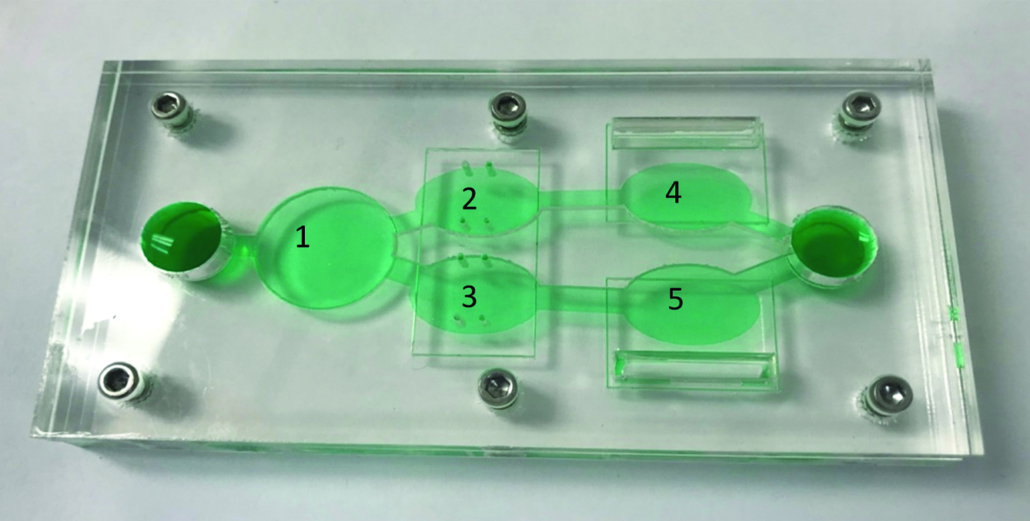
Hesperos Inc’s reconfigurable "body-on-a-chip" model simultaneously measures compound efficacy and toxicity, for both target cells and other organs, such as the heart and liver. The body-on-a-chip system contains five chambers that can house different populations of human cancer cells and organoids, some of which are grown on electrical modules (bio-MEMS), in a recirculating serum-free medium that mimics blood circulation. Christopher McAleer and colleagues reported that their microfluidic chip system can accurately capture the toxic effects of chemotherapies that have been metabolized by the liver – effects usually not seen in standard cell culture preclinical drug development or not reflected by preclinical animal models.
The researchers said, the results from in vivo testing with Hesperos Incs multi-organ-chip outperformed animal models in predicting the therapeutic index of several chemotherapeutics. The therapeutic index measures relative safety of a drug and the range in which a drug dose is determined to have a therapeutic effect before significant toxicity begins to occur. The initial determination of efficacy at the same time can currently only be done at the preclinical stage in animals, and animal models are not always a good guide to how a drug will perform in humans, Hesperos said in a press release.
The companies tested the device in two scenarios: on cancer-derived human bone marrow cell lines for anti-leukemia drug analysis, and on vulva and breast cancer cell lines to test multi-drug treatments in a multi-drug resistant cancer.
For the leukemia model, two bone marrow components were incorporated with liver tissue to measure the cytostatic effects of two anticancer drugs — diclofenac and imatinib — on bone marrow-derived cells and off-target effects on the liver. Testing showed that liver viability was not affected by imatinib, but was reduced by 30% with diclofenac.
In the second configuration, one multi-drug resistant vulva cancer cell line and one breast cancer cell line without multi-drug resistance were incorporated into the system with a liver compartment to determine metabolic effects, and with functional cardiac models to measure electrical and mechanical deficits from off-target toxicity. The common breast cancer drug tamoxifen reduced viability of the breast cancer cells only after being processed by the liver. Tamoxifen did not affect the vulva cancer cells except when co-administered with verapamil, a permeability-glycoprotein (Pgp) inhibitor. Both tamoxifen alone and co-administration with verapamil produced off-target cardiac effects, as indicated by a reduction of contractile force, beat frequency, and conduction velocity, but did not affect viability.
These results were consistent with what has been reported in human trials. But they were done in a lab, without the need of animal studies and with no risk to humans. Importantly, the system can be easily and quickly adapted by inserting new chips in different compartments, allowing for flexible testing of different organ systems derived from patient cells.


 ©FabienMalot
©FabienMalot Lonza Group
Lonza Group Vetter Pharma
Vetter Pharma