Better TNF? receptor blockers on the horizon
Two research teams have unraveled the complex dynamics of TNF? receptor signaling and have identified compounds that may be safer than current treatments.
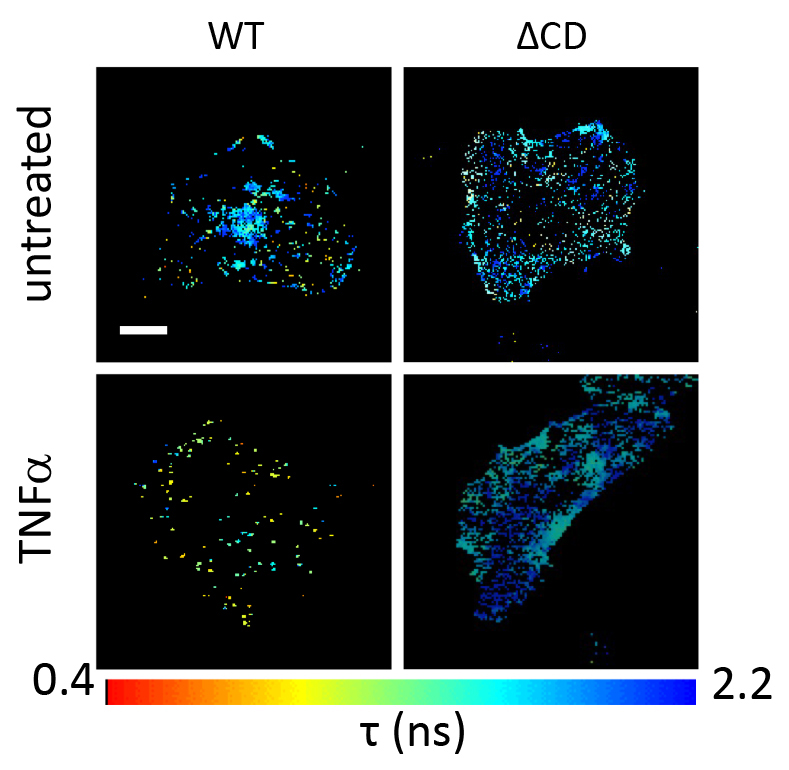
Penny Morton and colleages from Kings College in London uncovered how the organisation of the receptor for tumor necrosis factor ?(TNFR1) within the cell membrane triggers different functional outcomes, such as survival or inflammation, apoptosis or necroptosis by activation of gene expression downstream of the profinflammatory transcriptional regulator NF?B and and of AP-1. They confirmed that TNFR1 forms preassembled clusters at the plasma membrane of adherent cells in the absence of its ligand. However, after trimeric TNF? binding, TNFR1 clusters changed conformation, became more mobile within the cell membrane, interacted with the MEKK1 kinase, and activated the JNK/p38/NF?B pathway. These phenotypes required a minimum of two TNFR1-TNF? contact sites; fewer binding sites resulted in activation of NF?B but not JNK and p38. The new insights in TNFR1 signaling could lead to more targeted drugs that prevent severe side effects of current drugs that target the master controller of inflammation.
In a separate study, Chih Hung Lo et al. from University of Minnesota performed compound screening and identified a class of small allosteric molecules that perturbed the conformation of TNFR1. They observed that unlike previous TNFR1 inhibitors, their compounds efficiently blocked the ability of TNFR1 to activate NF-?B without affecting its binding affinity for TNF? or its assembly process. The researchers believe these compounds could be developed into TNFR1 inhibitors that are cheaper and less dangerous than established TNF?-targeting drugs.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink