Pharvaris BV raises $66m to advance oral HAE drug
Leiden-based Pharvaris BV received a cash injection that will be used to push PHA121, the very first orally administered hereditary angioedema (HAE) drug.
The Series B financing was led by Foresite Capital. New investors Bain Capital Life Sciences, venBio and Venrock, as well as the investors of a €15m Series A round in 2015 – Kurma Partners, LSP and Idinvest Partners – participated. Upon the financing, Brett Zbar (Foresite) and Richard Gaster (venBio) joined Pharvaris’ board.
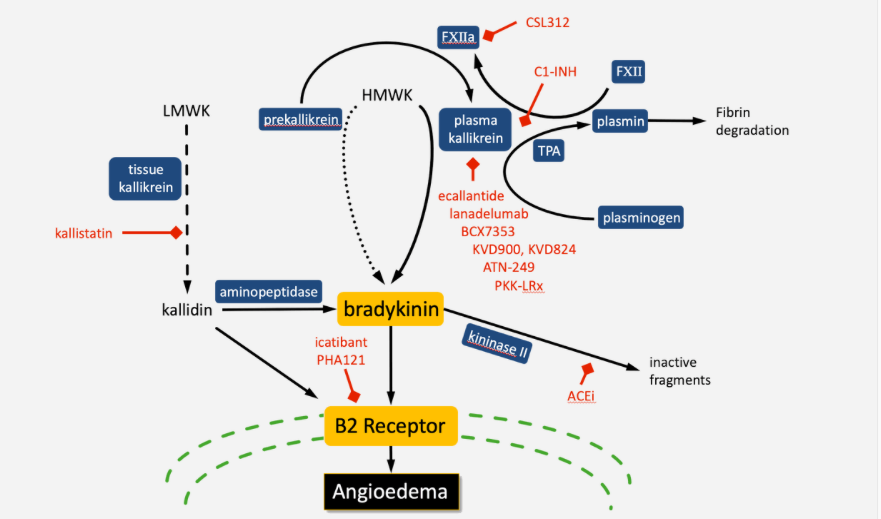
Pharvaris BV said it will use the assets to push clinical testing beyond ongoing first-in-man tests of its lead compound PHA121, an orally available bradykinin B2 receptor antagonist to treat hereditary angioedema (HAE) and related indications such as diabetic macular edema. The candidate is based on Fiazyr (icatibant), an injectible bradykinin B2 receptor antagonist marketed by Shire/Takeda, which took over Berlin-based Jerini AG in 2008 in a $521m deal. Pharvaris co-founder and ex-Jerini CSO Jochen Knoll is the inventor of icatibant.
All six marketed HAE drugs, that compensate the lack of C1 esterase inhibitor underlying HAE, are parenterally administered drugs. The oral compound PHA121 is hoped to offer an alternative to treat and prevent the autosomally dominant disease that is characterised by unchecked activation of the classic complement pathway and the bradykinin system.
Ex-Jerini AG CEO and Pharvaris co-founder Jens Schneider-Mergener recently took the helm as CEO at Hennigsdorf-based antibody developer Adrenomed AG, which is shortly before read-out of a potentially pivotal Phase II study in patients with septic shock.


 ©FabienMalot
©FabienMalot Lonza Group
Lonza Group Vetter Pharma
Vetter Pharma