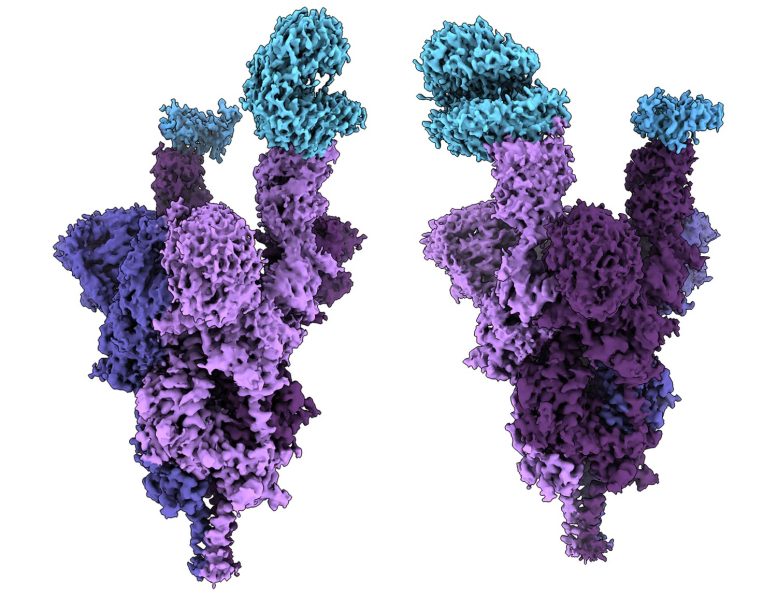
BioNTech and Pfizer evaluate Omicron-specific vaccine
Pfizer Inc and BioNTech SE have started clinical safety testing of an Omikron-specific vaccine in healthy adults 18 through 55 years of age.
The study will evaluate up to 1,420 participants across the three cohorts: In cohort #1 (n = 615), the participants enrolled have received full Comirnaty vaccination 90-180 days prior to enrollment and will receive one or two doses of the new Omicron-based vaccine . In the second cohor (n = 600), volunteers that additionally received a booster shot 90-180 days prior to enrollment will receive a fourth Comirnaty dose or one dose of the Omicron-based vaccine. In cohort #3 (n=205), unvaccinated participants will receive three doses of the Omicron-based vaccine.
The overall goal of the clinical trial ist to assess if an Omicron-specific vaccine will add any benefit to the patients. Independently from its outcome, Pfizer and BioNTech expect that they will deliver four billion COVID-19 vaccine doses in 2022.
To predict new variants of concern (VOCs) early on, BioNTech had partnered in 2019 with the London-headquartered machine-learning specialist Instadeep, who yesterday announced a $100m Series B financing led by led by Alpha Intelligence Capital; BioNTech and Deutsche Bahn AG. BioNTech’s and Instadeep’s AI-powered Early Warning System (EWS) for detecting high-risk SARS-CoV-2 variants identified more than 90% of World Health Organization designated variants on average two months ahead of time. It detected Omicron on the day its sequence became available among more than 70,000 novel variants discovered in October and November 2021.


 Vetter Pharma
Vetter Pharma International Journal of Molecular Sciences, doi: 10.3390/ijms17040561 JO - s
International Journal of Molecular Sciences, doi: 10.3390/ijms17040561 JO - s