Vector Biopharma kicks of with CHF30m Series A financing
With a Series A funding of CHF 30m from Versant Ventures, Vector Biopharma AG set out to solve several of the current gene delivery problems.
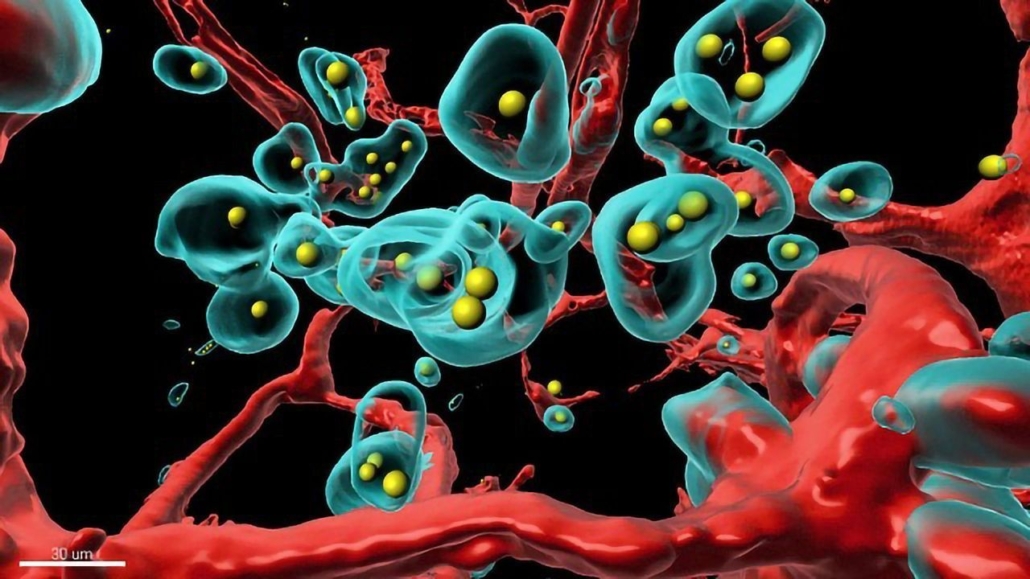
The company’s patent-pending Shielded Redirected Adenoviral (SHREAD ) platform, developed by antibody grandmaster Prof. Dr. Andreas Plückthun’s postdoc Dr. Sheena Smith at the University of Zurich, delivers gene therapy vectors that are only degraded in the liver by about 30 per cent after injection into the bloodstream as a result of a protein shell of trimeric scFv single chains that shields the adenovirus (AV5) vector from the immune system. The protein shield is interspersed with bivalent DARPINs which specifically target tumour stroma or tumour antigens, if present at the cell surface. According to Smith’s PNAS publication (doi: 10.1073/pnas.201792511), the vector can transfer 36 kb of DNA under regulation of a CMV promoter. The gene is expressed in the tumour microenvironment by the targeted cells, the resulting protein is glycosylated and exported directly at the site of action, which significantly increases the local drug concentration. In Her2-expressing xenograft models, Smith achieved excellent intratumoural trastuzumab levels. "This approach increases the tumour-to-bloodstream amount of a model antibody by 1,800-fold compared to direct administration," she notes in her PNAS paper. "Thus, this system could enable the local production of highly potent drugs with a greatly reduced risk of systemic toxicities." According to Vector Biopharma, its vectors can be produced in just three weeks using Plückthun’s so-called iMATCH technology for the production of HCAdVs. Vector Biopharma’s platforms can be used in particular for the development of drugs
– with a narrow therapeutic index, which are "too toxic" when applied systemically,
– such as costimulatory agonists or proinflammatory cytokines;
– such as biologics with unacceptably short half-lives or those that require a concentration gradient for immune cell recruitment, such as chemokines and cytokines;
– which in the mix aim to collectively remodel the tumour microenvironment and may otherwise have incompatible or non-overlapping toxicity profiles; and
– biologics that have highly complex post-translational modifications and are too costly to produce.
According to Plückthun, the current offering from the Swiss biopharma experts took ten years of research to make the platform work fine.




