Advanced formulations for biopharmaceuticals
In the past, the role of customised and tailored formulations have been largely underestimated in the field of biopharmaceutical product development. An advanced formulation strategy can strongly improve the product, leading to significant customer benefits. The growing and increasingly competitive biopharmaceutical market requires individual solutions, particularly for better product stability and prolonged shelf life.
Advanced and customised formulations not only enable the biopharmaceutical manufacturer to be more economical with even a higher product quality compared to competitor products but also help to generate intellectual property and to extend patent life span.
Customer benefits in focus
Advanced biopharmaceutical formulations enhance product stability, allowing the manufacturer to avoid a variety of additional indirect costs, which may include costs related to regulatory issues, brand risk, loss of product efficacy, back office invoicing, packaging, management time, patent safety, monitoring, and product recovery.
Achieving maximum product potential, which requires highest stability, is essential for the biopharmaceutical manufacturer. Improved stability through advanced formulations provides many advantages to manufacturers: i.e. allowing for higher temperature storage with self-applied biopharmaceuticals in pre-filled devices, for high concentrated subcutaneous application, or for the avoidance of lyophilisation steps. For all these approaches the cold chain aspect has to be considered. For example, it is a clear advantage for a manufacturer to provide products with longer shelf life, either when stored at 2-8 °C or even at ambient temperature. For instance, competitive advantages have been achieved by modified formulations that increased stability of therapeutic antibodies against psoriasis. Specifically, the antibody secukinumab (Cosentyx®) has a shelf life of only 18 months in the EU when stored at 2-8 °C. Advanced formulation by competitors resulted in a 24 month shelf life at 2-8 °C and was approved in the EU for storage up to five days at ? 30 °C (Taltz®) and in the US for up to 14 days at room temperature ? 25 °C (Siliq®).
Formulation matters
Currently, standards do not leverage the strategic opportunities of advanced formulation development and consider formulation too late in product development. This can delay development programmes, if product opportunities are not maximised prior to Phase II trials. Changes of formulations after Phase II may require additional studies, costs, and delays. Early product stabilisation and selection of a commercially viable formulation during manufacturing ensures shorter time lines and greater probability of success later in clinical studies and commercialisation. Multiple processing steps associated with mechanical, chemical, and temperature stress during drug substance and drug product development result in cumulating molecular changes. Therefore, tailoring the stabilising formulation by considering process-specific stresses should be integrated early in the manufacturing process, i.e. immediately after harvesting the biomolecule.
From gene-to-vial processing
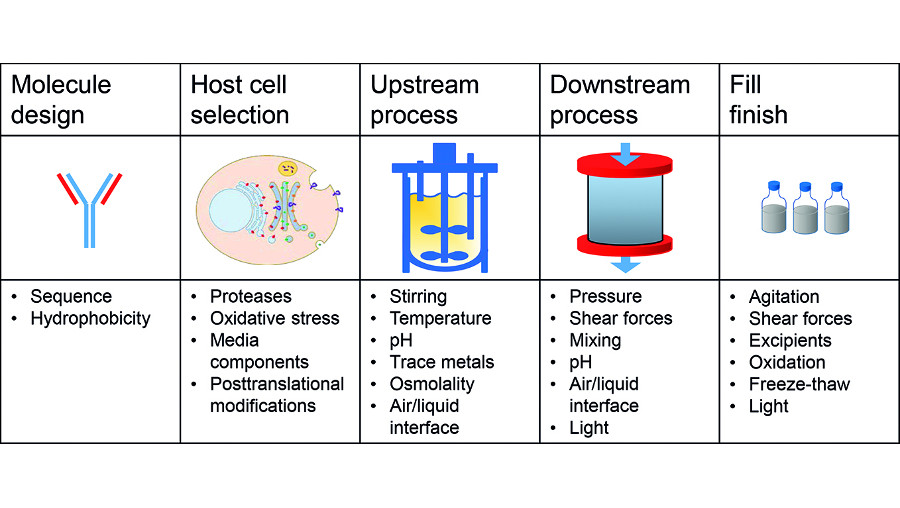
Current strategies of biologics manufacturers are aimed at increasing product quality. This already starts with molecule design and the selection of producer cell lines optimising the output while limiting side products such as protein fragments. But it also includes efforts in upstream processes through chemically defined media or tighter online monitoring. Similar efforts have been undertaken for downstream steps focusing on chromatography resins with higher specificity and resolution.
Particularly during the downstream manufacturing process, protein molecules are exposed to physical stress factors, such as stirring and mixing in conditioning steps, or shear stress in filtration operations or under high pressure. Chemical stress impacting protein stability is often connected to non-physiological pH values – for instance in low pH elutions from affinity columns at high protein concentrations. Both stress sources (Figure 1) can result in degradation or aggregation of the proteins, ultimately leading to loss of function or causing immunogenicity problems. High molecular weight aggregates can be eliminated during downstream processing. However, this will reduce the overall yield and the process efficiency by potentially requiring additional unit operations.
It has been shown in long-term stability studies that trace amounts of aggregates can act as nuclei for further aggregation thus limiting the shelf life of parenteral drugs in liquid formulation. As low-level product-related impurities are very hard to eliminate completely, other strategies, such as formulation optimisation to prevent the increase of aggregation during the shelf life, are strongly recommended.
Currently, most standard formulations are sufficient to support the typical short shelf life in clinical programmes. However, they are often not sufficient to support storage expectations for commercialisation. This means efforts should be undertaken relatively early to establish an advanced formulation and its implementation strategy to facilitate the delivery of competitive formulations. This holds particularly true in crowded indications or for second generation biosimilars where optimised formulations might give the competitive advantage to gain a larger market share.
SPS® in gene-to-vial processing
The SPS® formulation technology development platform is a best-in-class formulation platform for the stabilisation of proteins, like biopharmaceuticals, to intensify the development of better products. This includes improved product stability during refrigerated or even unrefrigerated distribution and storage, prolonged shelf life, as well as subcutaneous administration of high concentration liquid products etc. The sophisticated backbone of the SPS® technology platform is a library and database-based rational design approach. By means of this approach more than 100 different, regulatory well-known excipients are combined in order to select the most suitable excipient combinations, concentrations, and ratios for the individual biopharmaceutical product. The strength of the SPS® technology platform lies in the IP-protected amino acid-based formulation strategy in conjunction with the SPS® database, excipient library, and DoE support. All excipients used in SPS® are listed in relevant pharmacopoeias (USP, EP, JP, etc.) and/or as inactive ingredients by the FDA. According to the principles of preferential exclusion and preferential binding, SPS® formulations were already shown to be easily adaptable to a broad range of target molecules and requirements for dry and liquid products. The molecular integrity and functionality are the key analytic aspects to monitor the efficacy of the iteratively optimized and finally tailored SPS® formulations. As an example, SPS® significantly improved stability of the therapeutic IgG antibody trastuzumab during different processing stress conditions – i.e. accelerated aging – even in high concentration liquid formulation. SPS®-formulations avoided aggregation and fragmentation of trastuzumab even at concentrations ? 200 mg/ml and exhibited significantly reduced viscosity versus the original formulation.
Benefits of SPS®
The better product quality achieved by SPS® included in gene-to-vial processing and manufacturing helps the customer to realise significant competitive advantages in the market.
(First published in European Biotechnology, Autumn Edition 2017)


 Bayer AG
Bayer AG
 Picture from Ferdinand Stöhr on Unsplash
Picture from Ferdinand Stöhr on Unsplash