App design for the cellular processor
Since the earliest attempts at genetic engineering, scientists have sought to influence cell behaviour and development through the introduction of foreign genetic material. Synthetic biologists are now taking the idea to a whole new plane with app-like DNA-based software programs that turn cells into diagnostic sensors, therapeutic entities or tissue generators.
When Ron Weiss earned his Master’s degree in computer science at the Massachusetts Institute of Technology (MIT) back in 1994, the young researcher had no idea his work would reshape an entire field of scientific endeavour. He probably would have found it even more absurd if you told him back then that the field it would impact so dramatically was biology. At that point, he says, he stayed away from living systems because biology is so messy. But working with Tom Knight eventually changed his mind. The computer engineer and MIT professor was one of the first to attempt to control living systems through fundamental principles in engineering and programming. In his approach, DNA is biology’s software while the cells are the hardware – in other words, they should be programmable. Weiss quickly became fascinated with this notion that we might be able to program cells in the same way that we program computers. The enthusiasm from those early days has kept Weiss going through the many tough years since, as he struggled to design increasingly complex gene-based programs to alter messy biological entities – initially bacteria, then later mammalian cells. Today the researcher is unquestionably one of the world’s most highly respected experts in the field of synthetic biology.
Just as apps can turn smartphones on command into a device for communicating, translating, broadcasting, gaming or banking, the genetic circuits developed by Weiss are able to transform embryo-like cells into liver buds, detect and destroy infected or cancerous cells, or accelerate and improve drug development. And this is just the beginning, he says. Synthetic biology is moving past the basic research stage. More and more, pharmaceutical companies and investors are showing an interest. Synbio’-based companies raised US$500m during the first half of 2017 alone. It feels like the field is exploding right now, says Weiss.
Programming cells’, genetic circuits’, DNA software’ – after many years of arduous and frustrating experiments, the researcher knows that the terms used in synthetic biology are still little more than analogies. It turns out that it’s not that easy to program cells, says Weiss. We have to be humble in the face of biology, and appreciate how complex the situation is. Genetic circuits are generally made out of a set of between 3-15 genes, and that package of genetic building blocks is then inserted into a cell. What makes it different from classic genetic engineering is that a circuit is able to compute. It can sense what is going on inside or outside a cell – the level of a protein, a certain microRNA or mRNA, says Weiss. It then takes this information and computes like a miniature PC, which means it actually does something to the cell.
Cancer? We’ve an App for that
One type of program might eventually help fight the HIV retrovirus. The team led by Weiss at MIT have built a circuit able to detect whether a particular cell is HIV positive. From a computer logic perspective, that’s actually pretty easy, the researcher says. It’s a single input program. If the circuit senses a virus-specific protein like gag, it’s programmed to start a certain action – either killing the cell or inducing an immune reaction to it. Weiss says, however, that it’s still not clear whether the circuit is ready at this point to be developed as a therapy in its own right: We currently use it as a diagnostic tool to understand the dynamics of HIV. The ultimate goal is to build a circuit able to draw the immune system to tissues in an infected patient that hide latent HIV reservoirs.
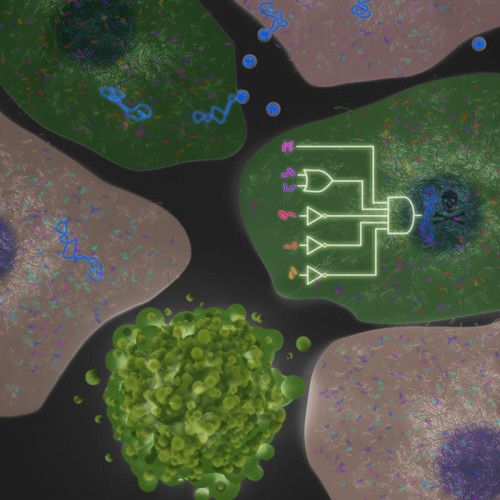
Another example of what genetic circuits could theoretically do is much more complex. Will they one day be able to detect whether a cell is cancerous, and act on the diagnosis? Here we’re moving from a single input to multiple inputs, because this is a complex disease, Weiss says. You almost never find a single biomarker for cancer. Systems that rely on just one biomarker, such as CAR T-cell therapies, often perform poorly. It’s believed that’s because they work by getting engineered T cells to lock on to a single surface receptor, usually CD19. But CD19 unfortunately doesn’t just appear on the cell membranes of tumour cells. Healthy cells can display it, too. CAR T cells can’t tell the difference, and wipes them out along with the diseased cells, which can have severe side effects for a patient. We need multiple inputs to determine with precision whether it’s a cancer cell or not, Weiss says. Specificity improves dramatically if a second receptor is added. We found that we need six inputs to get to a point where we think…it’s safe enough, and hardly ever makes a mistake.
The first genetic circuit able to distinguish between healthy and cancerous cells and switch on a killer protein garnered the group a publication in Science in 2011 (doi). But Weiss was aware then that although the data was exciting enough for a publication, it was far from mature enough for therapeutic applications. The circuit’s error rate was too high, he says. Genetic circuit outputs still have to be set up to provide one of two solutions – such as kill’ or don’t kill’. In this kind of setting, partially kill’ is not really ideal, Weiss says with a smile. It took his team five years to improve the circuit to the point where the system now makes only about one mistake in a billion cases. This we are ready to take into the clinic.
The circuit is ferried into patient cells via a herpes simplex virus (HSV). When it enters a cell, the first thing the circuit does is perform its computation, Weiss explains. And if the computation says yes, this is a breast cancer or a melanoma cell’, then the virus replicates and kills the cell. That sets free new viruses that subsequently infect other cancer cells. The researcher adds that although results from mice models have yet to be published, they indicate the system works. Initially the virus spreads throughout the entire mouse, Weiss says, but the circuit only acts within the tumour. He isn’t concerned about immune system responses to the therapeutic HSV. To the contrary: The circuit sends messages to immune cells and recruits them to act on the tumour.
Bugs in the program
A bigger problem is the length of the program’ necessary to make circuits function properly. The first version of the cancer-killing circuit was 5,000 base pairs (bp) long, but the final construct topped out at 40,000. More effective programs will certainly be longer – maybe much longer. But many viruses can’t carry that much genetic material. HSV’s limit is 150,000 bp.
As with other genetic technologies aimed at altering human cells, the delivery issue therefore remains a major hurdle. This is especially true for genetic circuits, which are usually much longer than gene therapy cassettes. The methods synthetic biologists use are no different from the methods developed in gene therapy, cell therapy and genome editing, says Yaakov (Kobi’) Benenson, a synthetic biologist at the ETH Department of Biosystems Science and Engineering in Basel, who collaborated with Weiss for years. The strengths and limitations of these approaches are carried over to any synthetic biology system intended for biomedical use, he adds. One way to ensure that a high percentage of cells in the body receive a desired genetic circuit is to modify cell samples ex vivo and retransplant them – a process that in many cases is already approved for clinical use. You can deliver relatively large packages of DNA ex vivo if you have time and can select correctly transduced cells, says Benenson.
 But accurate and effective systemic or localised delivery methods are still needed when delivering DNA inside the body, Benenson says: Large DNA payloads can be delivered by viruses with a lot of capacity – like gutted adenoviruses. But as the size of the DNA package grows, the physical size of the ferry – whether viral or non-viral – becomes more problematic. Larger ferries don’t diffuse as well, reducing delivery efficiency.
But accurate and effective systemic or localised delivery methods are still needed when delivering DNA inside the body, Benenson says: Large DNA payloads can be delivered by viruses with a lot of capacity – like gutted adenoviruses. But as the size of the DNA package grows, the physical size of the ferry – whether viral or non-viral – becomes more problematic. Larger ferries don’t diffuse as well, reducing delivery efficiency.
To address this issue, Benenson’s team has developed compression’ and decompression’ tools analogous to software programs that reduce (zip’) the size of files by transferring repeating elements – like those in promoters – just once. The compressed circuits are only reconstituted inside the cell (Nature Nanotechnology, doi). Elegant tools like this could help circumvent the delivery problems inherent in huge circuit therapies, but a lot of groundwork remains to be done. Treatments that require circuit transduction into patient cells in situ using systemic or local delivery, says Benenson, will take longer to develop.
Another issue is whether delivered circuits should integrate permanently into a patient’s genome. A transient localization in the nucleus, where a genetic circuit persists for just a few cell cycles, might in some cases be sufficient. And it would certainly be easier to get approval for that from medical watchdogs. If transfection is to be transient, DNA doesn’t even have to be the basic material used in the circuit. RNA would also be an option. Because its life is limited, delivering RNA would guarantee the circuit doesn’t become permanent, Benenson says. So it’s safer than DNA, which has a chance of permanent integration. Working with German mRNA-companies Biontech and Curevac, Ron Weiss is already experimenting with RNA-based genetic circuits. They deliver RNA that translates into a protein or peptide with a certain therapeutic asset, he says. The problem there is that RNA is constitutively expressed, and there’s no control.Any cell that the RNA goes into is going to make the protein. But there’s a way around this problem – by adding a programme that ensures the RNA is only expressed in the correct cell type. So you deliver to every cell, but it only expresses in particular cells or at a specific time, says Weiss. One drawback is that limited RNA lifetime could also pose a problem, e.g. if it decays before the circuit carries out its task. Excluding DNA from circuit components excludes a large repertoire of building blocks such as transcription regulators, says Benenson. So you must be able to operate with fewer possibilities. In the long run, he expects both approaches will have uses and proponents.
Growing new organs?
There’s no question that synthetic biology still has to solve a wide range of technical problems, but the possible rewards seem endless. Although they’re even more complex than the sense-and-kill designs that are being developed for HIV and cancer, some genetic circuits are able to steer cell differentiation in particular directions. To do that, programs have to react to a sequence of events that occur over time and in changing environments – where spatial, biomechanical and temporal interactions are taking place between different types of cells.
To guide differentiation of iPS or embryonic stem cells into target tissue types, developmental biologists currently add growth factors or other developmental triggers to cell cultures. Weiss isn’t doing that from the outside, but the inside: Implementing a genetic circuit within iPSCs provides a lot of power you just can’t have with external molecules. The circuits interact with a cell’s own genome, nudging it along particular differentiation pathways.
Starting with a homogenous mixture of iPSCs, the genetic circuit initiates differentiation, causing the cells to begin to self assemble and arrange themselves in patterns. Eventually they transform into the three germ layers – endoderm, mesoderm and ectoderm. If you are patient and wait seven days, says Weiss, the mesoderm begins to form vascular structures, and the endoderm differentiates hepatocytes that lead to a liver-like tissue. Other cells develop into hematopoetic cells or blood progenitors, while ectodermal cells can form brain buds’ and nerve cell conglomerates similar to forebrain tissue.
What Weiss calls synthetic morphogenesis is simple in theory, but difficult to achieve in reality. When his team started trying to coax iPSCs to develop into insulin-producing beta cells, the genetic circuit failed’ – and instead produced liver-bud-like tissue. It was a failure that turned out to be a spectacular success (Nature Communication 2015). The tissue the team acquired contains not just hepatocytes, but all the known cell types in a natural liver bud. And if the artificial tissue is grown in a 3D culture (as opposed to a flat, two dimensional culture in a petri dish) it develops into a miniature piece of liver that’s far larger than anything anyone else has been able to make so far. Weiss says that’s because the tissue also builds its own network of blood vessels, which allow oxygen and nutrients to penetrate deep into the bud. Other lab-grown organoids’ lack such vascularisation, which means they stop proliferating when they’re about a millimetre thick. The genetic circuits have created liver organoids that are multiple centimetres in diameter – 30 to 50 times larger than anything else out there, according to Weiss. And as the artificial tissue can be grown from iPS cells taken from a skin biopsy, it can be made patient-specific, and won’t be attacked by the immune system after retransplantation. We’re thinking now about transplantation studies in mice to see whether we can replace a liver.
But there’s more to do before Weiss and his team can publish the results of the 3D culture work: First, we really want to know how close this is to real biology. A cell analysis to compare transcriptomes of artificially and natural grown liver tissues is still ongoing.
In parallel, the lab is continuing to build a circuit able to generate beta cells. We put a genetic circuit into the cells that says: Once you become endoderm and mesoderm, take a right turn and become pancreas.’ Swiss colleagues in Martin Fussenegger’s synthetic biology group at ETH Zurich have already had some success at an endeavour like that. Their glucose sensor and insulin secretion circuit does essentially what a beta cell does, even though Weiss admits it’s still too slow. Naturally occurring beta cells can respond to changes in glucose levels nearly instantaneously. They make insulin and store it in vesicles, and if glucose levels rise in the surrounding cellular medium, they almost secrete it almost instantaneously. Fussenegger’s cells took tens of minutes to hours. Not quite fast enough, says Weiss. He now hopes to come up with a better beta cell app to either drive cell differentiation or use the circuit as a gene therapy that will re-enable the patient’s own beta cells to do their job.
Although that will take time, there are also more immediate applications for genetic circuits. The artificial human liver-buds, for example, are an ideal milieu for drug candidate testing. The organoid should be a much better predictor of what a drug candidate would do than an experiment in a mouse, Weiss says. And we can do all kinds of other cool things – like putting sensors in there – that can tell us in real time what’s happening. Such sensors could quickly sound the alarm if for example a drug candidate changes natural levels of crucial proteins, microRNAs or mRNAs. Weiss says tests with the common pain relief drug acetaminophen (paracetamol), which is toxic to liver cells in high doses, have already been a success: If we put excess acetaminophen on the liver bud, the sensors embedded in it fluoresced red, which told us that something was wrong. Benenson also believes circuits have the potential to provide much more information about drug candidates by reporting both intended effects and side effects. His lab developed comparable drug testing devices’ that are based on miRNA-targeting as a proof of concept, and now he intends to expand it to other target families like GPCR. This could shorten the drug-screening process by reducing the number of assays and counter-assays, he thinks. The researcher has already patented the methods, and is in talks with companies that want to use the technology.
Placing sensors in tissues shows one direction that synthetic biology will take in the future. Reproducing human tissues is another. But why should the field stop there? We don’t just want to replicate. We’d like to do better versions, Weiss says. Like a super-liver with even better metabolic activity. Maybe the next big thing made possible by cellular apps.
(First published in European Biotechnology, Winter Edition 2017)


 BioDlink
BioDlink
 Unsplash+
Unsplash+