Fighting AMR: turning the p(h)age
The rapid development of antibiotic resistance has become a major public health issue that could soon kill 10 million people annually. One promising alternative to ineffective antibiotics has been around for almost a century - bacteriophages. The microbe-killing viruses are used in medicine in Georgia, Poland and Russia, but have been neglected by western doctors. Now pressure is mounting on global health authorities to put the option back on the table.
When the 33-year-old Ukrainian soldier Dimitry F. badly injured his right knee during a mission, he had to be given a prosthesis. Before long, the area grew infected with a pan-resistant Pseudomonas strain – a potentially fatal diagnosis, because the bacteria was resistant to all currently available antibiotics. Only an old therapy from Georgia that was never really adopted by Western medicine could save his leg.
Bacteriophages, also known as phages, are viruses that target specific strains of bacteria and reproduce in them. In the process, they destroy their host so effectively they appear to have eaten it, which explains the Greek origin of the name (phagein = devour). After latching on to a bacteria’s outer surface, phages inject their own nucleic acid and hijack their host’s genetic machinery, effectively turning them into phage factories. Those factories eventually produce so many copies of the phage that they burst. If this process happens inside the human body, the phages produced eventually decay and disappear after lysing all of the target bacteria, while their components are metabolised. The viruses have been widely used for medical purposes in Russia, Georgia and Poland, but for a variety of reasons never took off elsewhere in the world.
Phages – an antibiotic alternative?
The Head of Septic Surgery and Infectious Disease Research at Charité University Hospital in Berlin has however built up a library of phages that specifically target the bacteria which produce biofilms. Last year, Andrej Trampuz founded the startup Phagomed Biopharma GmbH. Our vision is to develop phage therapy for indications where current antibiotic therapy is limited, says Alexander Belcredi, co-founder and CEO of Phagomed. The fact that we had so much knowledge about the experimental and last-resort treatments right from the start is a real asset. One indication Phagomed is working on is for periprosthetic joint infections like that contracted by Dimitry F. Bacterial infections of hip or knee prostheses are often associated with biofilms. According to Belcredi, the antibiotic dosages needed to eliminate that boundary and effectively destroy the bacteria are typically toxic for humans. Specific phage cocktails, however, could solve the problem. We’ve already demonstrated strong phage activity against S. aureus biofilms, Belcredi says. That allows us to eradicate a mature MRSA biofilm more effectively than with antibiotics, and at doses feasible in humans. The results of that study will be published this year. Phagomed was incorporated in November 2017, and is currently completing a seed financing round.
The problem of ineffective antibiotics in biofilm-associated infection is one aspect of a much wider problem – that antimicrobial resistance (AMR) as a whole is taking on frightening dimensions. Recently the World Health Organization published a comprehensive report based on data from 500,000 patients in 22 countries on the worldwide status of AMR. The numbers are alarming. Around 50,000 people are currently being killed by antibiotic-resistant infections every year in the EU and the US alone. The WHO estimates that annual costs generated by the problem top €7bn ($8.3bn) in Europe and $6.5bn (€5.5 bn) in the United States. New approaches for tackling the AMR crisis are therefore urgently needed. But do those approaches have to involve traditional antibiotic treatments? Phage therapy proponents say it makes sense to take a closer look at what’s already out there.
The first mass medical application of phages took place during the Winter War (1939-1940) between Finland and the former Soviet Union. During the three months of the conflict, around 6,000 soldiers were treated with bacteriophages. Big pharmaceutical companies like Eli Lilly (US) and Behring-Werke (Germany) also focused on the production of phage preparations in large scales at the time. But with the subsequent development of antibiotics, which can be used to treat a broad range of bacteria, phage therapy was soon ignored by pharmaceutical companies on the western side of the Cold War.
A long-forgotten history
The therapeutic advantages of phage therapy were first uncovered in 1917, when Felix d’Herelle was working as a research assistant at the Pasteur Institute in Paris. Although the researcher didn’t know what type of microorganism was killing the bacteria in his experiments, his findings opened a new chapter on tackling the infections they cause. In 1936, together with his friend microbiologist George Eliava, d’Herelle would lay the foundations for the George Eliava Institute of Bacteriophage, Microbiology and Virology (EIBMV) in the Georgian capital Tbilisi. The institute still exists today, and has been a last-ditch destination for patients with chronic infections since Western companies stopped phage programmes in favour of antibiotics. In Georgia, over 600 different phages are available in pharmacies in the form of special phage cocktails.
In Central Europe, Poland is the only country that employs phage therapy as a routine treatment for infection. At the Ludwik Hirszfeld Institute of Immunology and Experimental Therapy at the Polish Academy of Sciences (Wroc?aw), patients who have failed to respond to other treatments can receive phage therapy on compassionate use grounds.
In Europe, phage therapy isn’t illegal, but neither is it explicitly defined. The legal framework for the use of medicines in the EU – which is mainly dictated by a directive (2001/83) on medicinal products for human use that is over fifteen years old – does not address the question of phages. Doctors like Andrej Trampuz decide on their own whether a bacteriophage therapy should be allowed in special cases as a last-resort treatment option under the Declaration of Helsinki. Currently, no phage-based pharmaceutical is on the market in the US or EU, says Belcredi. Both the FDA and EMA are actively exploring how to regulate the therapeutic use of phages, but so far no phage product has been authorised for the market. To receive approval, quality manufacturing standards in particular have to be clarified and established.
The needle in a haystack
Phages are everywhere you find bacteria – in food, in puddles and pristine rivers, on computer screens, and of course on and in the human skin and gut. The viruses that prey on bacteria are about 100 times smaller than their hosts, and are thought to be the most abundant free-living entity on the planet. The number of phages all over the world is estimated at one hundred nonillion – 1032. Phages have co-evolved with bacteria for billions of years, which makes them highly specific to different strains of target bugs. The characteristic feature of bacteriophages to be more or less specific for bacterial strains within a single bacterial species used to seem a limitation of bacteriophage therapy, but in the era of resistance to broad-spectrum antibiotics, it now looks like a striking advantage, says Christine Rohde, a microbiologist at the Leibniz Institute DSMZ – German Collection of Microorganisms and Cell Cultures GmbH.
Phage therapy in Europe – getting the ball rolling
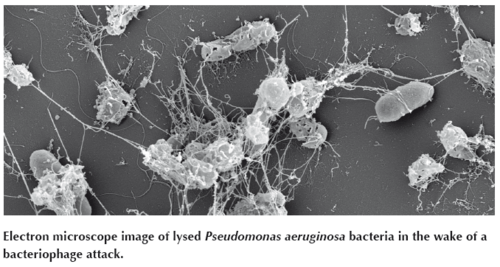
In September 2017, German joint project Phage4Cure kicked off an attempt to establish bacteriophage therapy within the framework of Western standards. In the project, researchers from the Fraunhofer Institute for Toxicology and Experimental Medicine (ITEM) and the Leibniz Institute DSMZ have come together with colleagues from Berlin Charité Clinic to attempt to get phages approved as drugs for bacterial infections. The project has received €4m of funding over three years from the German Federal Ministry of Research. The aim is to produce an inhalable product containing bacteriophages that fight infections with hospital-acquired Pseudomonas aeruginosa bacteria. The pneumonia it causes is one of the most common illnesses contracted during treatment in hospitals for respiratory, wound and urinary tract infections. Data from individual therapy applications of phages against P. aeruginosa has shown promising results.
Either phages with broad host coverages can be chosen and produced as sustainable pharmaceutical preparations for human application – for example in clinical trials – or phages might be specifically used in individual cases after a phage-host screening, says Rohde. Known as a phagogram, that screening is comparable to an antibiogram, with results returned overnight. That’s important for patients and treating physicians, because pathogens like P. aeruginosa multiply fast.
Phages attack only bacteria, leaving all other microorganisms and cells intact. That means treatments have very few side effects. The big downside of this specialisation, however, is that a limited number of strains within a bacterial species are targeted by a specific phage. According to Rohde, that’s a curse and blessing at the same time.
The microbiologist adds that preparations which have been imperfectly purified possibly posed the biggest danger when it came to worries about side-effects. In all, the DSMZ currently stores 800 phages that have been fully characterised. For the Phage4Cure project, Rohde and her team have already identified 70 potential therapeutic candidates. Among these are up to four very promising ones that will now be genetically screened and handed over to Holger Ziehr’s research team at the Fraunhofer ITEM. If you look at phage preparations from the past, they are often barely purified, says the ITEM Division Manager. For example, drug products commonly available in Georgia are often made up of microfiltrated supernatants, or have only been partly purified through ultrafiltration, explains Ziehr. But in the last few decades, he says, purification systems have matured so much that chromatographic methods can be applied to produce a pharmaceutical clean monophage. Fermenting bacterial hosts to make the phages per se is doable with batch processes.
Because phages naturally hem bacterial growth, there is also danger that resistance could evolve. That’s why the ITEM team’s long-term goal is to establish phage therapy as a platform technology. It should provide a range of monophages that can be easily interchanged in a phage cocktail that does not have to undergo a full approval procedure by medical agencies. Someday phage therapy might be treated like a vaccine. They’re updated every year as new flu strains emerge, says Ziehr. But the current challenges involved in applying existing pharmaceutical quality rules to phage APIs and the potential dangers of resistance could be dampening enthusiasm among potential investors, who are still cautious about investing in phage research.
The first small steps
The EMA has not yet published any guidelines for the production of phage therapeutics. Before that can happen, the efficacy and safety of phage therapy in general has to be demonstrated. A promising step towards the legal establishment of phage therapy as an alternative to antibiotics was achieved in 2009 with the foundation of the Brussels-based international NGO called P.H.A.G.E. (Phages for Human Applications Group Europe, www.p-h-a-g-e.org). Its goal is to increase research on bacteriophages and initiate programs to introduce them as an approved therapy in Europe.
Three years ago, the European Union was also willing to spend some attention and money on phage therapy. It contributed €3.8m to the first industry-standard Phase I/II Phagoburn clinical trial, which evaluated phage tolerance and efficacy in fighting sensitive antibiotic-resistant infections. For the trial, researchers from France, Belgium and the Netherlands recruited 220 burn victims whose wounds had become infected with the common bacteria E. coli or P. aeruginosa. The trial was divided into two parts – 110 patients for each of the two bacteriophage cocktails. Each targeted a specific strain of bacteria, and contained 12-13 monophages identified and developed by French company Pherecydes Pharma. Its COO Jérôme Gabard has great hopes for the firm’s products. Phagoburn received all the necessary authorisations in France, Switzerland and Belgium – proof of the quality of our phage therapy method and the development invested in the tested products. The trial is ongoing, and Gabard says results are expected in a few months.
 Pherecydes has raised €8.7m in a Series B financing round led by Go Capital. With this financial support, the company intends to produce phages for compassionate use and TUA (Temporary Authorization of Use) in accordance with GMP. We also want to move two programmes into the clinical phase in 2018-2019 for infections with S. aureus and P. aeruginosa respiratory tract infections, says Gabard. The company is looking at building its own pharma production unit in Nantes to produce bacteriophages at an industrial scale according to GMP guidelines. In Europe, just two production sites are currently approved to produce phages in line with existing pharmaceutical good manufacturing practices: one in France (Clean Cells SAS) and the other in Slovenia (AmpliPhi Biosciences Corp.).
Pherecydes has raised €8.7m in a Series B financing round led by Go Capital. With this financial support, the company intends to produce phages for compassionate use and TUA (Temporary Authorization of Use) in accordance with GMP. We also want to move two programmes into the clinical phase in 2018-2019 for infections with S. aureus and P. aeruginosa respiratory tract infections, says Gabard. The company is looking at building its own pharma production unit in Nantes to produce bacteriophages at an industrial scale according to GMP guidelines. In Europe, just two production sites are currently approved to produce phages in line with existing pharmaceutical good manufacturing practices: one in France (Clean Cells SAS) and the other in Slovenia (AmpliPhi Biosciences Corp.).
And after decades of ignoring phage therapy, the market isn’t only moving in Europe. Two weeks ago, US-based Intralytix, Inc. received FDA clearance to initiate Phase I/IIa clinical trials targeting adhesive invasive E. coli (AIEC) pathogens in patients with Crohn’s disease. Intralytix currently has the world’s largest portfolio of FDA-approved phage-based products on commercial markets. In 2015, it entered into a collaboration agreement with Ferring Pharmaceuticals (Switzerland) to develop a proprietary and well-defined set of bacteriophages specifically designed to treat inflammatory bowel diseases.
Dimitry F. survived. After five days and periodic treatments with a phage cocktail (Pyo-Phage) his wound healed and he left the hospital on two legs. In the EU, phages can still only be used if there are no other alternatives. And the patient still has to be told that the phage cocktail is not an approved product. At least – not yet.


 eXmoor Pharma
eXmoor Pharma
