Making every cent in testing count
It's no easy task to develop medications that are effective and safe to use. The pharmaceutical industry loses billions every year due to safety-related attrition during the drug discovery process. For both patients and companies, improving productivity in the area would bring significant benefits. Now novel biomarkers designed to better detect and manage drug-induced organ injury - both preclinically and clinically - could make the process faster, safer and cheaper. tg
Did you know that in Britain alone, up to 500,000 patients every year are hospitalised after taking medications – like acetaminophen – that are generally considered harmless? 500 of them will die from toxic effects to the liver. In the US, the same medicines are responsible for an estimated 2,500 deaths per week. Taking properly prescribed drugs is the country’s fourth most common cause of death.
The problem is that serious drug safety issues often occur only rarely, meaning drug developers can easily miss them until late-stage clinical testing or right before market authorisation. Matters are complicated by the fact that our ability to monitor areas like drug-induced organ damage remains limited. The biomarkers that are currently available aren’t sufficiently sensitive, specific or predictive. In worst-case scenarios, this leads to potentially life-saving drugs having to be withdrawn at the last minute, ruining development work that has often gone on for more than a decade – it takes an average 13 years for a new medicine to make it from bench to bedside. And then the huge development costs have to be written off.
Even a Phase III failure can mean a waste of €1bn or more, says Dr. Michael Merz. The industry expert in preclinical and clinical drug safety from the University of Zurich is coordinating a five-year project aimed at identifying drug-induced safety issues much earlier in the drug development process – ideally during preclinical or Phase I development. Even if it has only moderate success, the €28m TransBioLine project that was launched by the Innovative Medicines Initiative (IMI) in April 2019 could have a huge impact. Analysts estimate safety issues in the industry lead to costs in excess of a hundred billion dollars annually.
Drug safety: a complex task
Overall, 20% of all clinical trial failures – and more than 65% of post-launch withdrawals – have been attributed to clinical safety issues like organ-based, mechanism-based or off-target toxicity. A recent analysis of the attrition of 812 candidates developed by AstraZeneca (AZ), GlaxoSmithKline (GSK), Pfizer and Takeda revealed that non-clinical toxicology was by far the highest cause of attrition, accounting for 40% of drug failures, while Phase l safety issues contributed to a further 25% of failures. The industry experts stressed that although minimising safety-related attrition has been a significant area of investment across the industry in the past decade, it remains a key area for improvement that could only be addressed by collaboration and development of new assays tackling the complex problem. In 2014, the FDA estimated that just a 10% improvement in the ability to predict drug failures before clinical trials could save US$100m in development costs per drug.
According to Dr. Joanne Bowes from Global Safety Assessment at AstraZeneca, what makes the problem so complex is that types of adverse drug effects ADEs) vary broadly. About 75% are dose-dependent and principally predictable from primary, secondary and safety pharmacology. The other 25% of (mostly lethal) ADEs, however, include unpredictable idiosyncratic responses, dose-related ADEs, long-term adaptive changes and delayed rebound effects following discontinuation of therapy.
From target selection
Roughly two decades ago, Big Pharma companies realised that datasets from individual companies were too small – and therefore insufficient – to solve the drug safety issue. For the first time, they began to share data and collaborate precompetitively. Two FP6 pilot projects coordinated by the European Federation of Pharmaceutical Industries and Associations (EFPIA) – dubbed InnoMed, AddNeuroMed and InnoMed PredTox – ended with the formation of the IMI in 2008, which has attracted €5bn in funding so far from the EFPIA and the European Commission.
Current industy strategies for improving the management of safety issues are two-tiered. On the one hand, drug developers try to sort out drug candidates that might show mechanism-based or off-target safety problems as early as possible in the drug discovery process.
An over-reliance on animal models of disease has in part led to poor levels of Phase II survival.
Along with CROs, companies like Pfizer, Takeda or GSK now reportedly apply so-called pharmacological promiscuity indices’ to exclude potentially problematic compounds that activate safety relevant targets – such as the cannabinoid receptor CB1, a well-documented inducer of suicidality. With new tools like CRISPR editing, RNAi and knock-outs, they have identified dozens of such targets that pose safety concerns.
In the end, successful pharma R&D comes down to careful target selection, says Torsten Hoffmann, VP of Drug Discovery at Taros Chemicals GmbH. His company coordinates the European Lead Factory (ELF), an EU drug-discovery resource launched by the IMI in 2013. In May, it received €36.5m in funding for the ESCulab project, which is aimed at finding new targets and compounds via high-throughput and phenotypic screening against the 550,000 compounds currently archived in the Joint European Compound Library.
Drug discovery companies have also adopted machine learning, and they can now give compounds a functional impact score based on gene ontology annotations, transcriptional profiles and off-target sites across different species. Drug discovery CROs or pharma companies have also benefited from MetaMapTox, the world’s largest metabolomics database, which was developed by Biocrates Life Sciences subsidiary Metanomics Health. It documents over 100 validated toxicological modes of action in several rat organs.
to translational biomarkers
The other great hope of drug development companies is to identify and validate translational biomarkers that are sensitive and specific enough to assess drug-incuded organ injury in both animal models and healthy volunteers in early-stage clinical trials.
An important task of preclinical safety research is to identify the doses at which new compounds cause adverse effects. So we need biomarkers with well-established performance characteristics, says Jan Hengstler, Head of System Toxicology at the Leibniz Research Centre for Working Environment and Human Factors (IfADo) in Dortmund. Numerous useful biomarkers of toxicity have been established in the past. Therefore, the challenge of current research is to clearly demonstrate whether new biomarkers show superior performance metrics in a specific context. According to Merz, existing biomarkers like creatinine or ALT are generally not sensitive enough to detect drug-induced organ injury before the organ has already been damaged significantly. That has driven the search for novel biomarkers that work in both animal models and humans. A development programme is often killed if toxicity issues are seen preclinically, even though there is no proof that ADRs that occur in animals will also occur in humans. Technologies that have emerged in the past few years now offer new options for establishing such improved translational biomarkers, or are bridging the predictability gap with human-cell screening.
According to Torsten Hoffmann from Taros Chemicals, the most promising emerging and bridging technology relates to human organ-on-a-chip systems, in which biomarkers are used to to establish a therapeutic index. Such systems use reprogrammed human induced pluripotent stem cells (hiPSCs) or organoids embedded in a matrix that mimics the physico-chemical conditions in organs (such as beating cardiomyocytes), and compartments are connected through microfluidic channels.
Within the FP7 and Horizon 2020 frameworks, IMI consortia led by Roche and Pfizer have invested €90m to establish the quality-controlled hiPSC cell banks StemCellBanc (2012, Basel) and EBiSC (2014, Cambridge, UK/Saarbrücken, Germany). They’ve also set up differentiation and manufacturing protocols for human cells in secondary drug screening. Consortia of EFPIA member companies have also played prominent roles in validation studies aimed at establishing novel biomarkers for drug-induced organ damage.
A decade ago, the FDA described the situation as follows: Three organs need better clinical monitoring of drug-induced injuries. They are:
- Kidney: current standard biomarkers increase only once 50-60% of kidneyfunction is lost.
- Liver: standards are not sensitive or specific enough, and do not adequately discriminate adaptors from patients at high risk of developing liver failure.
- Vascular System: currently no biomarkers available for drug-induced vascular injury in humans.
Within the IMI pilot Innomed Predtox, 15 pharma companies and two SMEs identified damage markers such as KIM-1, CLU or TIMP-1 for drug-induced kidney injury (DIKI) by means of transcriptome and proteome analysis. New biomarkers were added by the Predicted Safety Testing Consortium (PSCT), which was established in 2006 under the supervision of the FDAs Critical Path Institute. In 2008, its first candidate biomarkers were passed to the FDA for qualification. The work was continued and extended to biomarkers for drug-induced liver injury (DILI) and vascular injury (DIVI) – which contribute significantly to clinical attrition due to safety problems – by the IMI’s Safe-T consortium (2009, budget €36.5m). It was headed by Novartis and AstraZeneca, and coordinated by Merz.
The IMI’s prominent role
In 2018, the FDA qualified six renal injury biomarkers for use in Phase I studies: albumin, b2 microglobulin, clusterin, cystatin C, KIM-1, total protein and trefoil factor 3. Within the new IMI project TransBioLine, qualification will be expanded for DIKI markers, completed for DILI and DIVI markers and started for pancreatic and CNS injury markers. Merz stresses it will be crucial to continue TransBioLine activities even after the end of the funding period. In fact, head-to-head studies have recently identified novel biomarkers that reflect kidney and vascular function independently of inflammation related to organ injury. In studies on 30,000 patients, proenkephalin (penKid®) was non-inferior to glomerular filtration rate, the in vivo gold standard for kidney function assessment. A marker called adrenomedullin (bio-ADM®) has been shown to reflect endothelial dysfunction in humans. Another kidney-specific early injury biomarker, uromodulin, has just been opened for licensing.
Latest approach: miRNA
Since research teams across the world recently discovered that tissue-specifically expressed micro-RNAs can hint at organ injury in earlier stages, research teams have tried to establish circulating miRNA profiles from blood samples as tissue- and mechanism-specific diagnostic tools. The hope of the TransBioLine contributors is to find profiles in banked blood samples that can be used to monitor ADRs and diseases like NASH. According to Merz, this would not only help weed out unsuitable drug candidates early in the development process, but also improve risk assessment and therapy management of effective drugs that have proven safety issues.


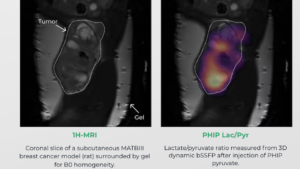 Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany
Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany Sitryx Therapeutics
Sitryx Therapeutics