Controlled human malaria infection trials
Tackling the malaria pathogen P. falciparum has been complex, due to rapid development of resistance. A new compound targets liver stages of the parasite and, thus, provides a new mode of action to reduce the population of the parasite.
The use of human challenge trials (HCT) or controlled human infection modelling (CHIM) has been endorsed by governments since the 1940s, when the Common Cold Research Unit (CCRU – 1946 – 1989) at Salisbury utilised an ex-American Red Cross Hospital left behind by the War Office after the European conflict for challenging healthy volunteers with rhinovirus.
Building on induced infectious disease modelling in healthy volunteers and utilizing many of the inoculation and monitoring procedures identified when malaria infection was employed as a treatment for neurosyphilis in the 1900s (malariotherapy), the first Controlled Human Malaria Infection (CHMI) or Volunteer Infection Study (VIS), as we now know it, was undertaken in 1949, in order to trial the efficacy of quinine derivatives.
The malaria model has two common procedures for initiating infection. The most true-to-life method involves inoculation of sporozoites via a mosquito bite (or by a direct injection of sporozoites under the subject’s skin). The per-cutaneous inoculation of sporozoites allows both liver- and blood-stage infection to develop, whilst the second procedure, direct venous inoculation with erythrocytes parasitised with Plasmodium, results only in blood-stage infection.
CHMI has primarily been used to look for novel targets for malaria therapy (basic research) and for proofs of efficacy (PoE). It has been argued that CHMI does not take into account the boosting effect that anti-malarial vaccines may provide to those with preexisting immunity (antibody and T-cell); however, this has not been evidenced, even in the large scale roll-out of the first licensed malaria vaccine: RTS, S/AS01.
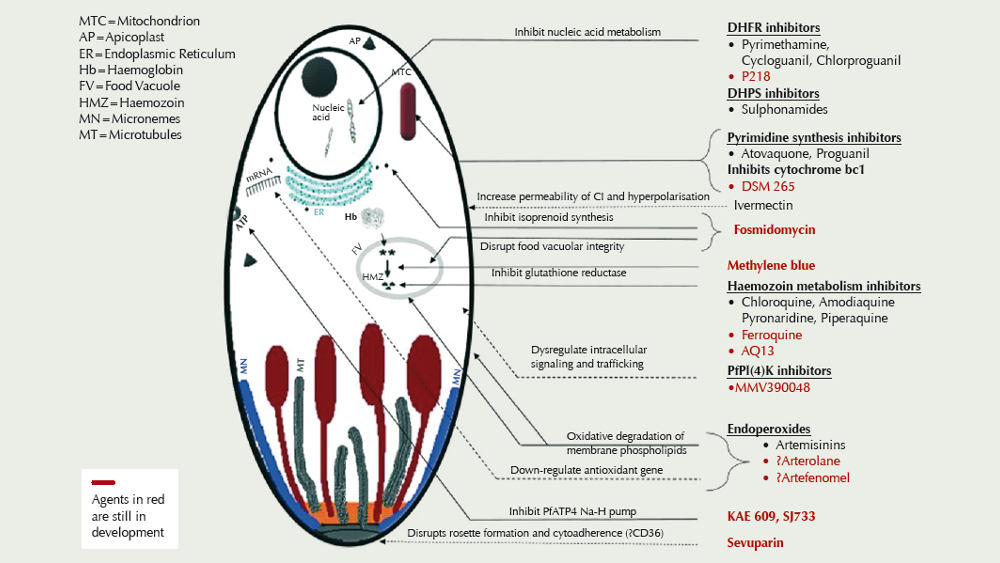
There are currently some 13 compounds in development (see figure), driven in part by resistance of Plasmodium falciparum to the artemisinin derivatives, piperaquine, and mefloquine in Southeast Asia.
Towards a long-acting therapy
SGS, as a leading CRO, recently commenced enrolling healthy volunteers for the first Malaria Volunteer Infection Study (VIS) in Belgium, on behalf of Medicines for Malaria Venture (MMV).
The primary objective of the early phase trial is to measure the efficacy of the experimental MMV compound in killing malaria parasites in the liver before they can develop and reach the bloodstream, thus protecting people from malarial disease. It is expected that those people living in P. falciparum endemic areas, primarily central Africa, will benefit from a new, affordable chemoprotective agent. Previous chemoprotective studies have demonstrated the efficacy of low-dose primaquine in breaking the cycle of transmission. A new drug, capable of blocking infection, could reduce the number of carriers to a level where the natural, Anopheles spp. vector-mediated transmission is broken.
First published in European Biotechnology Magazine, Summer 2019 edition.




 Sartorius AG
Sartorius AG