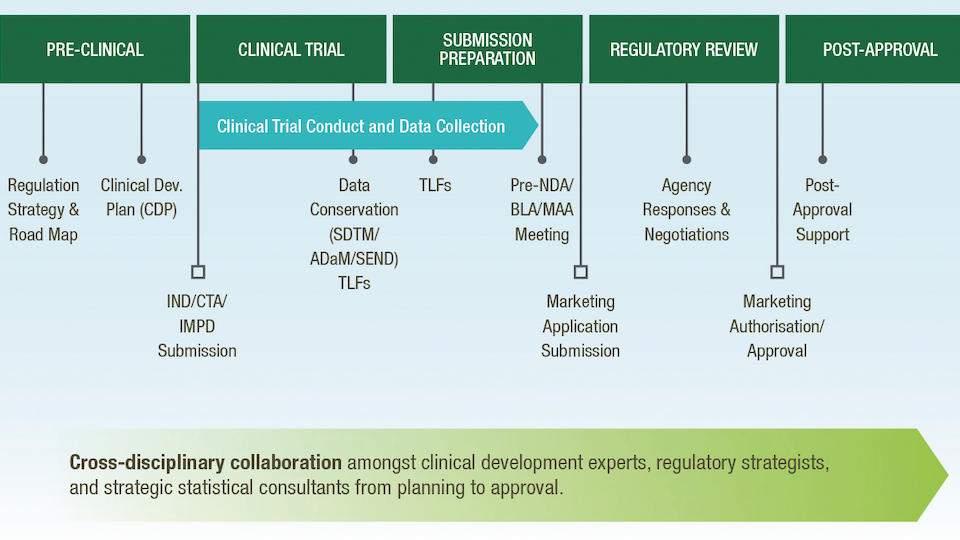
Wholistic approach to strategic consulting
Strategic planning for the development of a novel therapy is critically important to reduce financial burdens, save time, and find the best, shortest path forwards. No two products will have the same pathway or same criterion for optimisation, but early, forward-looking planning can result in greater efficiency and a better understanding of key decisions and timepoints along the course of novel therapy development.
Strategic consulting is the contribution of cross-disciplinary questions, ideas, and experience that increases critical thinking and forward planning towards the study and development of a novel product.
Early clinical development
Strategic input is critical in constructing the clinical development plan (CDP), a document that details the proposed pathway from an initial clinical study through eventual marketing application with key decision options outlined to the extent possible. This document typically includes proposed indications, planned study design(s) based on the competitive landscape and other similar products and indications, proposed sample sizes, options for study adaptation, and the narrowing or expanding of the study population, as appropriate. The CDP is a living document that can be updated with increasing knowledge gained during the development phase with contributions made by regulatory strategists, clinical operations, and strategic statisticians. With insights from experienced strategic team members, the CDP can detail the long-term plan for product development, providing visibility and opportunity to identify go-no-go’ decision timepoints and efficiencies during development.
Go-no-go decisions are predefined timepoints and criteria for a decision to either continue product development, modify the pathway, or stop development as currently planned. The timing and criteria should be prespecified with consideration to all possible pathways along a decision tree to ensure that products with promise are continued and risks for continuing the development programme, including cost, timeline, and likelihood of success, are well understood. The decisions are impacted by multiple disciplines, and the related risks should be considered by all parties, requiring the merging of identified clinically meaningful results, appropriate endpoint selection with appropriate statistical analysis methods and consideration for Type I error control, key requirements or objectives from regulatory agencies, and consideration of the timeline and competing landscape with other products. Go-no-go decision planning is critical early in development to ensure that appropriate efforts are directed to products most likely to lead to marketing authorisation.
Meetings with regulatory agencies can be planned to occur at opportune times when the clinical development team can receive valuable input to help direct the pathway towards a more efficient route for eventual marketing application and approval. As a partnering document to the CDP, a target product profile (TPP) is an organisational, planning, and communication tool to present the minimally acceptable vs ideal results for the planned indication, patient population, treatment duration, modes of administration, and standards for clinical efficacy. In the early stages, the TPP can be used as a concise, early presentation of the ultimate goals of the product development to inform conversations with regulatory agencies to ensure agreement surrounding the development plan.
Regulatory interactions
Regulatory interactions and feedback should be considered carefully in the context of the greater clinical development programme. Changes to study design, endpoints, eligible patient populations, or study procedures, can have an impact on the generalised conclusions that can be made from the clinical programme. In general, Veristat recommends early conversation on key planning elements with regulatory agencies. This can be expedited with key elements identified through the careful development and updating of a CDP.
Early protocol development
An early opportunity for strategic input is during development of the first-in-human or first-in-indication protocol. Strategists can advise on:
- Selection of objectives and endpoints for establishing safety and initial demonstration of efficacy
- Identification of surrogate endpoints and/or biomarkers for prognostic or predictive effect
- Alternative study designs, including adaptive or Bayesian methods that might reduce the time on study and exposure of subjects to the novel treatment at sub-therapeutic levels
- Appropriate sample size selection with context to available information on potential safety and toxicity of the product and relevant conclusions for proof of efficacy
- Optimal pathways for progressing the clinical development pathway to support later regulatory requirements
Early in product development, the key to success is to think ahead – an optimal solution needs not only to be a good decision today, but also to advance the product effectively and efficiently through the CDP without wasting time or patients.
Rare diseases
For rare diseases, particularly where there are no, or few, alternative treatments, strategic input is critical to identify an appropriate study design. Strategists can help overcome:
- Small sample sizes
- Lack of medical experts/sites
- Limited info/consensus on standard of care
- Access to patients
- Difficulty identifying clinical outcomes to measure
- Lack of control or comparator data
- No disease-specific endpoint validation
With only a limited number of patients available worldwide, an efficient approach must consider the total number of patients required for each study, as well as the use of appropriate controls. The gold standard clinical trial design is a double-blind, randomised, controlled trial; however, this is not typically feasible with low patient populations worldwide. When possible, the use of non-concurrent or synthetic control arms can be beneficial. This, of course, requires early and strategic interaction with regulatory agencies.
In some settings, these control arms may not be available, and single-arm non-comparative studies can be used as the key evidence for marketing application and approval.
Decisions regarding study design in this context are closely entwined with the appropriate treatment of patients and the regulatory strategy.
The cross-disciplinary considerations of statistics, clinical operations, clinical development, and regulatory strategy must be considered to create an optimal and feasible approach to product development.
Conclusion
Many factors contribute to an optimal strategy, including the type of product, indication(s) of application, disease prevalence, and the competitive landscape. This is why strategic planning from an experienced, cross-disciplinary team of experts is critically important for the efficient and effective development of novel therapies.
Early, forward-looking planning, the development of tools, such as the clinical development plan and target product profile, frequent interactions with regulatory agencies, and consideration of nuanced details that may be most effectively handled through non-standard clinical trial design, can result in a more efficient pathway to market.
This article was originally published in European Biotechnology Magazine Winter Edition 2022.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink