AAV Gene Therapy: Monitoring Manufacturing
Adeno-associated viruses (AAV) have revolutionised gene therapies as safe and effective gene shuttles. The growing demand for AAV vectors now requires an industrialised process throughout pre-clinical development that involves comprehensive monitoring from production to patient to ensure safety and efficacy of the therapeutic agent and reduce cost of goods.
The gene therapy sector is booming: According to the American Society of Gene and Cell Therapy, the number of clinical trials has grown exponentially since 2014 with 16 gene therapies being already approved. Gene therapy, including genetically-modified cell therapies, already account for 53% of all therapies in development. The great majority of projects in the pipeline leverage viral vectors for gene delivery, with AAV playing a central role as a versatile and potent virus model.
While the AAV field has mastered the challenge of engineering customised safe and effective viral vectors in the past decade, it is now struggling with establishing and optimising an industrialised production process that meets the fast-growing demand. Currently, there is a real shortage in vector supply and many CDMOs and CROs are now entering the field to fix this, states Katja Betts, CEO of PROGEN. The German biotech company, founded in 1983, is an established partner for AAV analytics covering all steps of R&D and manufacturing with proven techniques and an exclusive portfolio of reagents.
High quantity in high quality
Viral engineering determines the genetic strategy of the vector by individual plasmid design. Optimal gene design is not the only important factor for therapeutic success. The viral capsid that carries the vector to a designated cell or tissue greatly influences the efficacy of the gene transfer and the immunogenicity of the shuttle. Katja Betts is excited to see the wealth of opportunities that novel capsids bring to the rapidly developing space of AAV vector design.
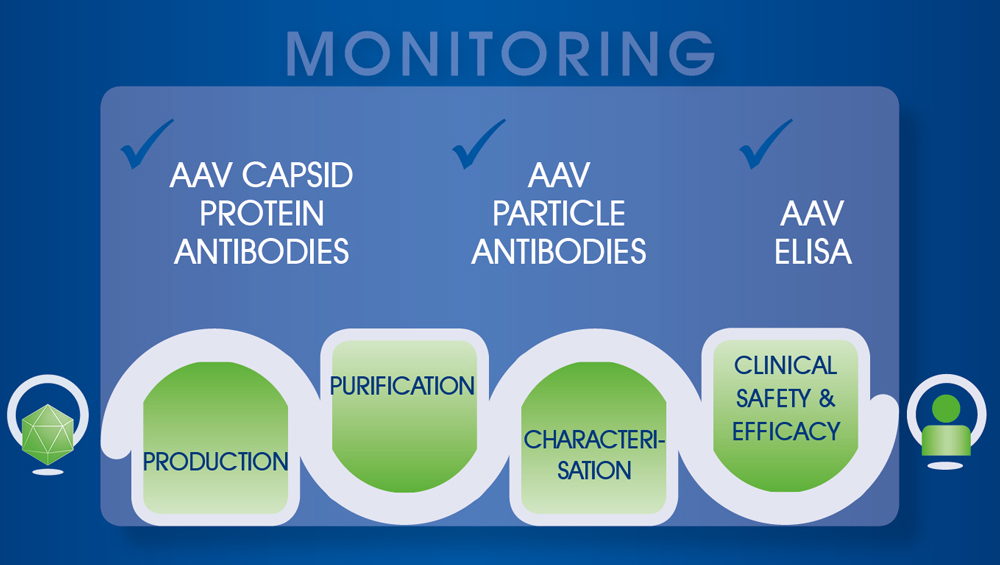
The early stages of AAV vector development involve the optimisation of the plasmid and its packaging along with the set-up of an expressing stable cell culture. For pre-clinical and clinical manufacturing, the system has to be upscaled for the production of high quantities, including an adaption of several production and purification parameters. This critical phase requires comprehensive monitoring of all steps and their careful optimisation to control identity, titer, purity, and potency of the vector (see figure). One of the biggest manufacturing challenges is to obtain a high yield of fully assembled capsids with high potency in the expression of the transgene while reducing cost of goods. Another requirement in pre-clinical development is the quantitative determination of AAV vector titers as a basis for correct dose assessment.
Proven analytical assays like western blot or ELISA, based on AAV capsid protein antibodies or AAV particle antibodies are fast and reliable monitoring tools for every step of the process. They are used to purify, analyze and characterise the intermediate products, ensuring optimal performance of the manufacturing system. Increasingly automated use promotes process consistency and standardisation, e.g. in regulated pre-clinical and clinical settings. In addition, pre-screening of patient sera for AAV-neutralizing antibodies improves clinical safety and efficacy.
With its analytical portfolio of AAV antibodies, ELISAs, and standards, PROGEN will continue working with the gene therapy field to advance AAV manufacturing, e.g. by increasing production speed, optimizing sample volume, automation & standardisation, and reducing costs.



 Roche
Roche