Seed money for unique topical hair growth inducer
Mallia Therapeutics GmbH has raised seed money to accelerate preclinical research and start Phase I testing of a baldness cure with unique MOA named soluble CD83.
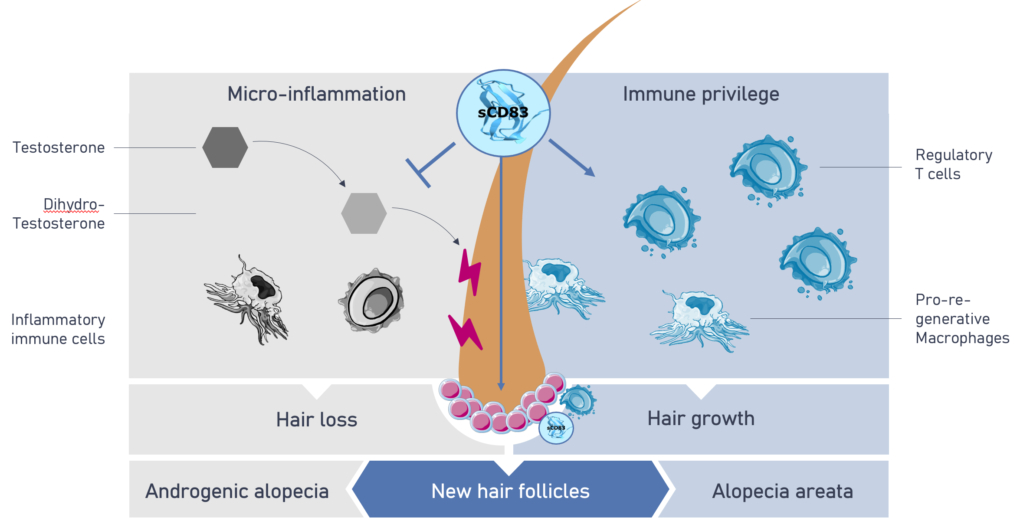
Good news for all those with genetic hormonal hair loss (androgenic alopecia and Alopecia areata): according to the patent specification, an anti-inflammatory soluble peptide called sCD83 may not only help patients with arthritis, diabetic foot ulcer or poorly healing wounds, but it also makes the hair on bald heads grow back – at least a little in mice. sCD83 prevents the dihydro-testoterone-induced micro-inflammation of hair follicles and subsequent hair loss in favour of boosting regulatory T cells and pro-regenerative macrophages leading to hair growth. The PR effect, which already won €500,000 at the m4 award two years ago, led to the founding of Mallia Therapeutics GmbH (former working name MalliaBiotech) in Erlangen and now a seed financing in an undisclosed amount – undisclosed, presumably because the IZB Pitch Day is approaching these days in Munich and Mallia is allowed to present there in front of 40 investors, including Hightech-Gründerfonds (HTGF), who supported the company’s presentation.
The company, spun off in June and led by Institute Director Alexander Steinkasserer (Univ. Erlangen) and Manfred Gröppel is based on the work of Dmytro Royzman and published a European patent two years ago that covers the application areas of anti-inflammation and hormonally induced hair loss – the latter affects around 50% of hairless people. Unlike previous products, the sCD83 formulation stimulates the formation of new hair follicles.
Worldwide, over 50% of men as well as 50% of postmenopausal women are affected by hormone-induced androgenetic alopecia (pattern baldness) and another 147 million suffer from autoimmune-mediated alopecia areata. Current treatments do not induce new hair growth but only extend the life cycle of existing hair or work to systemically suppress the immune system with associated adverse effects including severe infections. Mallia expects to address both these shortcomings with sCD83 with a superior safety profile and a dual mechanism of action to preserve hair and induce new hair follicles: Firstly, it induces an anti-inflammatory environment at the hair follicle via regulatory T cells (Tregs), which interact with follicular stem cells and thereby activate hair growth. Secondly, sCD83 directly binds to follicular stem cells where it induces the formation of new hair follicles.
In a presentation at the EHRS Conference, the co-founder and scientific director of Mallia Therapeutics Dmytro Royzman presented data from a preclinical androgenetic hairloss model showing that sCD83 accelerated the hair growing phase (anagen phase)and induced new hair growth. In addition, using a human ex vivo system, sCD83 treatment prolonged the growing phase of human hair, and hair growth-associated pathways are induced. Further, sCD83 application led to the expansion of a stem cell population within the human hair follicles.
According to Managing Director Manfred Groeppel, Mallia Therapeutics is going to use its seed funding to bridge the phase until closing of a Series A round maybe this year. “Given the promising results of our ongoing preclinical studies including results using tissue samples from patients, we hope to move into the clinic and start treating patients within the next two years, he said.