VectorY Therapeutics raises €129m in Series A round
Dutch VectorY Tx BV has closed one of this yers's largest Series A financing rounds in Europe at €129m to push its vecorised antibody pipeline in CNS proteinopathies.
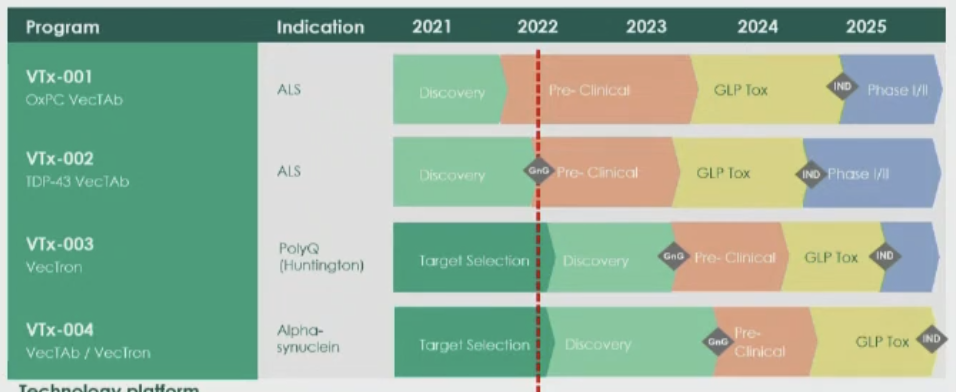
EQT Life Sciences and Forbion co-led the €129m Series A financing that will support clinical development of VectorY Therapeutics BVs lead programme VTx-002 in Amyotrophic Lateral Sclerosis (ALS), and preclinical development of VTx-003 in Huntington disease as well as further programmes in early development stage. Both programmes are based on VectorY’s vectorised antibody platform in proteinopathies that constists of a structurally engineered AAV5 vector with modified capsid and the transgene that carries the instructions for a cell to make the therapeutic antibody. In connection with the financing, Wouter Joustra, General Partner at Forbion, Arno de Wilde, Director at EQT Life Sciences, and Karin Kleinhans, Partner at MRL Ventures Fund, will join VectorY’s board of directors.
New and existing investors also participated in the financing, including MRL Ventures Fund, Insight Partners, the ALS Investment Fund, Forbion Ventures, BioGeneration Ventures (BGV) and an unnamed business angel.
VTx-002 is the company’s vectorised antibody programme delivered to the brain using a one-time modified AAV vector with high CNS tropism that can cross the blood-brain barrier. Upon brain expression, the antibody targets TDP-43 for the treatment of ALS. In most people with ALS the TDP-43 protein is misfolded and forms aggregates in the cytoplasm of motor neuron cells. This is problematic for two reasons. Firstly, it leaves too little functional TDP-43 in the nucleus. Secondly, the buildup in the cytoplasm is toxic. The combination of too little nuclear TDP-43 (loss of function) and toxic cytoplasmic aggregation (gain of toxicity) is thought to contribute to loss of motor neuron cells in ALS. According to the company, VTx-002 selectively clears misfolded and aggregated TDP-43 from the cytoplasm of neurons thereby restoring the function of TDP-43 in the nucleus leading to preservation of neuronal cell function and health.
Our program is uniquely positioned to address TDP-43 pathology, which underlies the disease in the vast majority of ALS patients, said Sander van Deventer, CEO of VectorY Therapeutics. The company uses an AAV5 manufacturing platform that gives “high yields at low price”.



 Freepik.com
Freepik.com University of Geneva
University of Geneva