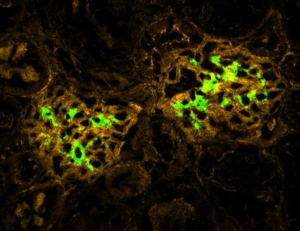
Microbiotica’s microbiome therapy showed 63% remission in ulcerative colitis phase 1b trial
Cambridge-based Microbiotica’s ulcerative colitis (UC) candidate met its primary and secondary phase 1b study objectives on safety, efficacy signals and bacterial engraftment. The company has reported positive results from a small, placebo-controlled study of its oral precision microbiome therapy MB310 in patients with mild-to-moderate ulcerative colitis, adding a rare positive clinical signal in a disease area where microbiome approaches have so far struggled to gain traction.
In the 29-patient trial, 63% of patients treated with MB310 achieved clinical remission, compared with 30% in the placebo arm. The readout comes as many microbiome-based therapies have struggled to demonstrate clear and durable clinical benefit beyond early-stage studies in inflammatory bowel disease, with several programs failing to translate promising signals into later-stage success. Following these results, Microbiotica plans to advance MB310 into larger trials to find out whether its approach can deliver more sustained benefit in ulcerative colitis.
A closer look at the data
MB310 is an oral live biotherapeutic composed of a consortium of eight commensal bacterial strains, “each selected for their significant association with improved clinical outcome in a faecal microbiota transplantation (FMT) clinical trial,” added the company’s CEO, Tim Sharpington. The therapy is taken once daily and is intended to be administered alongside standard-of-care treatment in patients with ulcerative colitis.
Beyond the headline remission rate, Microbiotica is pointing to a combination of follow-up data and biological readouts.
“Firstly, it was very pleasing to see that the bacterial strains within MB310 engrafted within a week of the start of dosing and were maintained for the three months that followed the end of dosing. Secondly, the histological changes associated with MB310 treatment confirmed our pre-clinical findings and showed strong improvements of markers of mucosal damage,” commented Sharpington in an email to European Biotechnology Magazine.
Indeed, following the trial, all MB310-treated patients who entered follow-up were reported by the company to have achieved sustained clinical remission. In ulcerative colitis, disease activity can fluctuate, and placebo responses are often high, particularly in small, early-stage trials.
The company also reported changes in objective readouts alongside symptom improvement. These included improvements in histological assessments of colonic tissue and reductions in faecal calprotectin, biomarkers commonly used to monitor inflammatory activity in ulcerative colitis, alongside symptom-based endpoints in UC trials.
Microbiotica also reported rapid and sustained engraftment of all eight bacterial strains contained in MB310 throughout the 12-week dosing period and into follow-up. Achieving consistent engraftment has been a recurring challenge for microbiome-based therapies in inflammatory bowel disease, where colonisation does not always translate into durable biological or clinical effects.
Why microbiome approaches in ulcerative colitis have struggled
A number of companies have attempted to translate microbiome science into therapies for ulcerative colitis, with mixed and often disappointing results. Early clinical interest centred on FMT, which showed that altering the gut microbiome could induce remission in some UC patients. However, outcomes across trials proved variable, and responses were often difficult to sustain once treatment stopped, limiting enthusiasm for FMT-derived approaches outside academic settings.
Efforts to industrialise and standardise microbiome therapies have faced similar challenges. Seres Therapeutics advanced its oral microbiome product SER-287 into a phase 2b study in mild-to-moderate UC, but the programme failed to meet its primary endpoint despite evidence of bacterial engraftment. More recently, Vedanta Biosciences pursued a defined bacterial consortium approach with VE202, which also did not meet its primary endpoint in a phase 2 UC trial, leading the company to deprioritise the indication.
These programmes have faced recurring hurdles, high placebo responses in UC trials, inconsistent alignment between symptom improvement and objective inflammatory markers, and the difficulty of translating bacterial engraftment into durable clinical benefit.
Other companies besides Microbiotica remain active in the space. Earlier this year, Belgium-based MRM Health obtained FDA clearance to proceed with its candidate MH002 phase 2b trial for UC. The phase 2a involved 45 patients with mild-to-moderate UC, and MH002 was reported to be safe and well tolerated, with modest improvement in endoscopic severity compared with placebo, significant improvements in stool consistency from about two weeks, and an 18 % clinical remission rate at eight weeks in the treatment group versus 0 % with placebo. Data also suggested anti-inflammatory effects, including reductions in faecal calprotectin.
What 2026 looks like for Microbiotica
It is a challenging space Microbiotica is venturing into with MB310, but the results add to the limited number of positive clinical signals in UC. According to Sharpington, if those results were to be confirmed in larger studies, MB310 has the potential to alter the narrative in UC and provide long-lasting disease remission.
The company is now looking into the design for a phase 2/3 trial that will explore the disease-modifying potential of MB310. “Decisions on dosing regimens and concomitant medication will be investigated in future studies. In the acute phase of active disease, we anticipate this therapy will be given in combination with anti-inflammatory and/or immune modulatory induction agents. The goal would be that our precision microbiome medicine maintains patients in remission for longer periods of time,” said Sharpington.
Beyond clinical progress, the company said in its press release that in 2026, it will explore partnering and financing options to determine the best route to fund its products through later studies and towards commercialisation.


 MAES-AREA-Englos
MAES-AREA-Englos