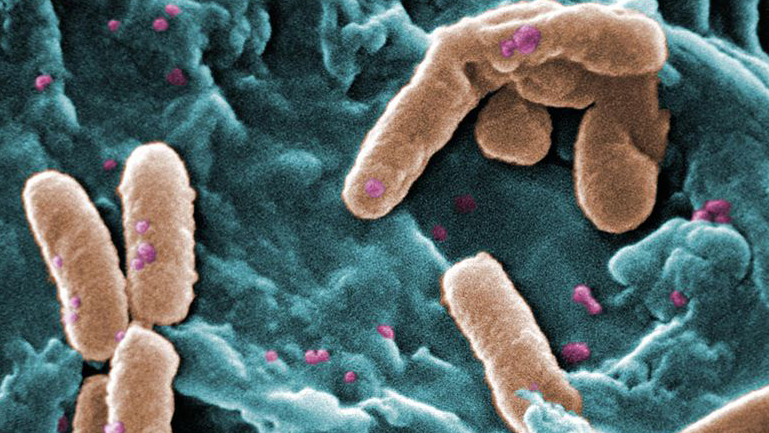
Is AMR the next, but hidden, crisis?
With the coronavirus pandemic mounting, experts are already warning of antimicrobial resistance as being the next, hidden crisis. The AMR community still urgently waits for substantial pull incentives. While the US DISARM act was pulled at the last minute from the country’s corona stimulus package, the UK’s NHS has started stakeholder engagement for its flat-rate model. Meanwhile, Forge Therapeutics has closed a €170m deal with Roche and Paratek received a long-term BARDA contract.
Typically, antibiotic work has not been seen as a high priority for big pharma in the past years, but with COVID-19, this may change. At the end of March, Swiss pharma company Roche announced a deal worth up to €170m with US-based Forge Therapeutics to work on a therapy for antibiotic-resistant Gram-negative bacteria, including Pseudomonas aeruginosa, which can cause lung infections. This deal adds up to the $5.7m CARB-X grant awarded to Forge in 2019. “We look forward to combining our novel approach with Roche’s proven drug development and commercialization expertise to provide a truly new class of antibiotics,” says Forge CEO Zachary Zimmerman.
Additional good news was provided by US-based Paratek at the end of 2019. The company received a five-years contract from Biomedical Advanced Research and Development Authority (BARDA), with an option to extend to 10 years. Under the agreement, the development of Paratek’s Nuzyra® for the treatment of pulmonary anthrax is supported, as well as FDA post-marketing requirements associated with the initial Nuzyra approval, and the option to procure up to 10,000 treatment courses of the drug for the Strategic National Stockpile. “We are honoured that BARDA has selected us. This provides us with enough capital through the end of 2023,” CEO Evan Loh told European Biotechnology. However, in light of other companies going bankrupt (Archaogen, Melinta) or facing an acquisition (Tetraphase), Loh points out that “one company doesn’t make a sector successful.”
He and many other experts in the field are emphasising, that antimicrobial resistance might be the next global, but silent, pandemic crisis. “As we come together to fight today’s COVID-19 crisis, we must also look ahead to the next one. We cannot be short-sighted, and we cannot be complacent, especially about antibiotic resistance,” urged Julie Gerberding, Chief Patient Officer at MSD, in a recent article. In addition, there is growing concern about how COVID-19 deaths are linked to AMR (See BMJ 2020;368:m1091 or this Lancet article or this summary of articles). “Secondary infections are often way more deadly than the original infection. We need an armamentarium of functioning anti-infectives,” comments Aleks Engel, investor from the REPAIR Impact Fund on the need to fill up the antibiotics pipeline to combat AMR.
Manica Balasegaram, a medical doctor who heads the Geneva-based Global Antibiotic Research and Development Partnership (GARDP) also urges that while antibiotics won’t treat viruses directly, they are an important line of defence against secondary bacterial infections, like ventilator-associated pneumonia, urinary tract infections, and sepsis, which are more common with prolonged stays in intensive care units, particularly among people with weak immune systems. “We don’t have a line of sight into the supply and demand of antibiotics right now. But, in outbreaks, you still need essential medicines like antibiotics,” says Balasegaram (see his full statement here). This is underlined by first studies from Chinese COVID-19 patients, which showed that those who recovered from severe cases mostly received antibiotics.
This wouldn’t be a major concern if it weren’t that the antibiotics market is already suffering from years of neglect, resulting in a dry pipeline of novel antibiotics and a broken business model. "Neglecting for years the investment in the prevention, treatment, and control of infectious disease has now led to a predictable global crisis,” Marc Gitzinger, Vice President of the BEAM Alliance and CEO of Swiss BioVersys stressed. “While we now have to focus on the emergency measures, I hope that we learn some lessons. Society should value again the R&D of vaccines, antibiotics, and diagnostics for infectious disease.”
From this background, effective pull incentives to encourage development and reimbursement of novel antibiotics are in high demand, but countries are still moving slowly. In March, the UK Government, along with NICE and the NHS England, started a stakeholder dialogue around the details of their pilot approach. Thus, the NHS England proposes to pay a subscription fee of up to £10m/year for a decade for each of two new antibiotics covering WHO priority pathogens, whether the antibiotic is used or not. According to AMR expert John Rex, ”the analysis suggests that they are proposing – if the rest of the G20 pays their fair share – a global pull incentive of up to $4bn.”
And, what are other countries doing? In the US, experts such as Kevin Outterson, executive director of CARB-X, are pushing forward US Congress to pass the Developing an Innovative Strategy for Antimicrobial Resistant Microorganisms (DISARM) Act (see this article, among others). There was some hope that the DISARM Act could be included in one of the national COVID-19 stimulus packages, but that did not happen so far. According to Evan Loh, another emphasis should be to join forces across Europe. “Neither the country-by-country pricing system nor EMA’s need for extra data compared to FDA requirements makes it easy for antibiotic SMEs to survive post-approval,” he says. “It’s time for everyone to work together.”
This article was published in the European Biotechnology Magazine Spring Edition 2020 released on 9th April 2020.
Interested in get connected with global AMR experts? Please join the AMR Conference yearly organized by BIOCOM AG. Being a platform to connect relevant AMR stakeholders from industry, SMEs, finance, hospitals, science, and policy, the congress will offer the opportunity to discuss solutions for solving the long-term AMR challenge and, thus, being better prepared for future pandemics. www.amr-conference.com