Digital solutions to serialisation
Though blockchain technology is still far from maturity and mainstream adoption, 22% of life sciences companies surveyed by Health IT Analytics in 2017 said they would experiment with the technology. Four years from now, 80% of them believe, blockchain usage will be widespread.
In contrast to current centralised data hubs that store 2D barcodes, including the serial number, batch/lot number, expiry date, and global trade item number printed on medicine packs, blockchain technology is not centralised but works with a distributed database network (Fig. 1). Within the network, every change or manipulation can be seen in real-time by every stakeholder who has the permission to participate in the network.
This allows forgery-proof, tamper-evident tracking of a (pharmaceutical) product‘s lifecycle, even as it changes hands between the manufacturer (pharma company), logistics service provider, wholesaler (distributor), retailer (pharmacy), and consumer (patient). Every single action (i.e. sending or repackaging a vial from a certain lot to a distributor) is signed with a digital fingerprint and time stamp and broadcasted to the trusted members of the private network. Simply spoken, blockchain is a data format that uses previous entries in a sequence as part of the current entry: an algorithm attributes a unique “block“ to every single action, which is calculated from the chain of existing blocks and then added to the growing chain, creating a unique data set that can’t be altered by single parties.
Prototypes launched
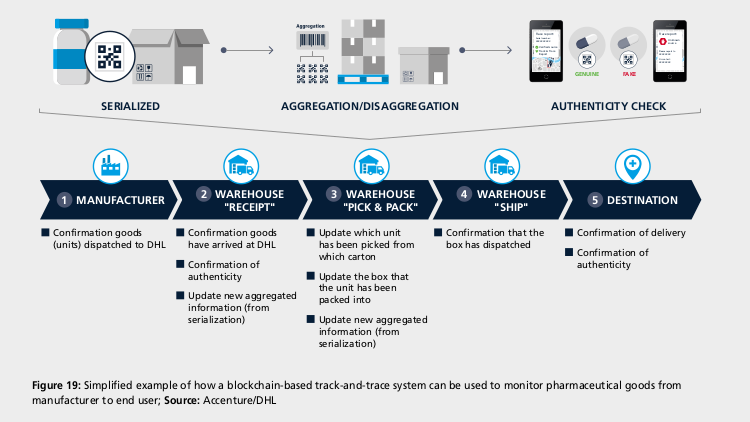
A key challenge in pharmaceutical serialisation that can be solved by blockchain technology is maintaining traceability when units are repackaged or aggregated from unit to case to pallet for logistics purposes and then disaggregated back down to unit level for consumption. A few solutions are in the advanced stage:
In March, DHL and Accenture established a blockchain-based track-and-trace serialisation prototype. In a simulation, the system was able to track more than 7 billion unique pharmaceutical serial numbers and over 1,500 transactions per second in a global network of “nodes” across six countries. According to Keith Turner, CIO at DHL Supply Chain, “we are refining the solution … with key industry stakeholders to operationalise the concept.”
Nascent market
Database giant SAP, along with 15 Big Pharma companies, is working on a prototype solution that uses the MultiChain protocol, an early blockchain based on Bitcoin’s technology. According to Raimond Gross, Chief Blockchain Strategist at SAP, it provides the “only production grade infrastructure available,” it‘s “easy to use,” and “everyone knows its founder Gideon Greenspan.”
As the US Drug Supply Chain Security Act (DSCSA) requires the pharma industry to adopt an “interoperable system” to manage records of ownership and transfers of prescription drugs in the United States, San Francisco-based startup Chronicled, Inc. announced last March its MediLedger project with data privacy and business intelligence would meet the requirements of the pharma industry (a proof-of-concept study was carried out with SAP customers Genentech, AmerisourceBergen, McKesson, and Pfizer) and was fully compliant with the DSCSA.
According to speakers at TraceLink‘s Futurelink conference in Munich this spring, blockchain technology is private by nature, as it runs decentrally and thus does not attribute accountability to a legal entity, such as the US Food and Drug Administration (FDA) or European Medicines Agency (EMA). Futhermore, blockchain is not compatible with the EU general data protection regulation (GDPR), because it does not allow users “to revoke the use of personal data at any time.”
For the moment, blockchain has had a long run in terms of industry-wide adoption for serialisation. Other applications, such as the management of real world data with predefined data protection settings, may drive adoption in the future.